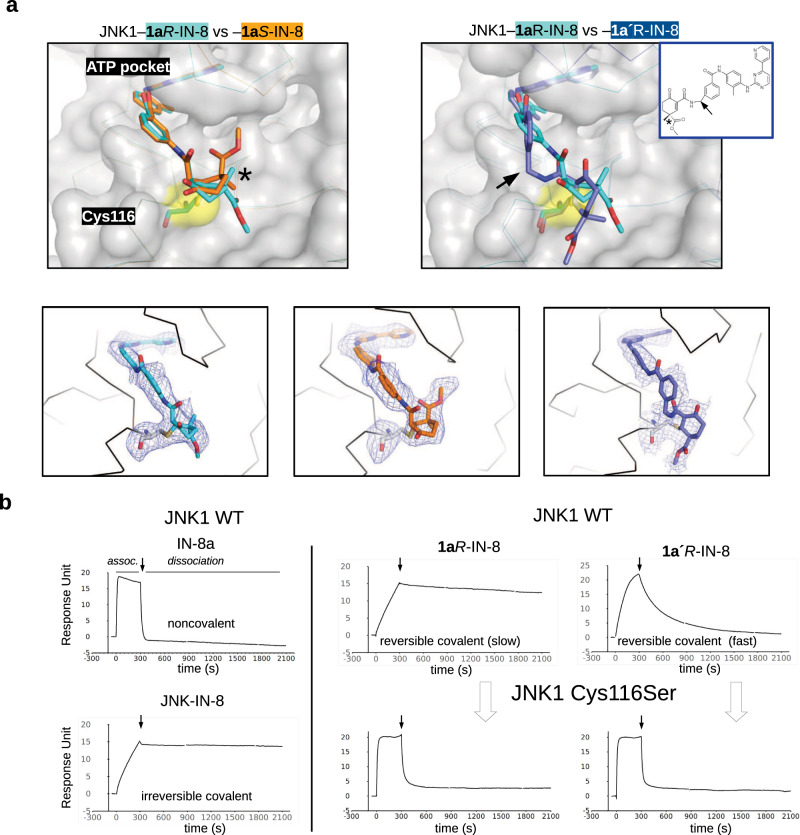
Fig. 2. Crystal structures of covalent inhibitors bound to JNK1.
a Structural comparisons of JNK1–1aR-IN-8, –1aS-IN-8, and –1a’R-IN-8 crystallographic complexes (with the following newly deposited PDB IDs: 8PTA, 8PT9, 8PT8, respectively). These show that the C4 stereogenic center could be used to direct the carboxymethyl group towards different directions in the substrate binding pocket next to Cys116 (left panel), or an additional methylene between the ATP-pocket binding moiety and the warhead necessitates a dramatically different conformational solution to form the cysteine covalent adduct (right panel). Lower panels display the Fo-Fc omit map for the cysteine-small molecule covalent adduct contoured at 1.5ϭ. b Validation of the cysteine mediated covalent bond by SPR experiments. Mutation of Cys116 to serine increases the kinetic dissociation rate validating the importance of covalent bond formation upon binding. Panels show the results of surface plasmon resonance (SPR) experiments with IN-8a (acetylated IN-8; noncovalent), JNK-IN-8 (irreversible covalent), 1aR-IN-8 (reversible covalent, slow dissociation) and 1a’R-IN-8 (reversible covalent, fast dissociation) binding to JNK1. Note that the association phase (assoc.) of the SPR curve is determined by the kon, while the dissociation by the koff. Compounds were injected over the JNK surface for 5 min at a concentration corresponding to their ~KD and the dissociation of the JNK-compound complex or adduct was monitored in time (the start of the dissociation phase is shown with an arrow). Note that the kinetic binding profiles of 1aR-IN-8 or 1a’R-IN-8 on the JNK1 C116S surface resembles that of IN-8a (the ATP-pocket binding moiety without the warhead) on the wild-type (WT) JNK1 surface, suggesting that Cys116 is indispensable for the decreased koff.