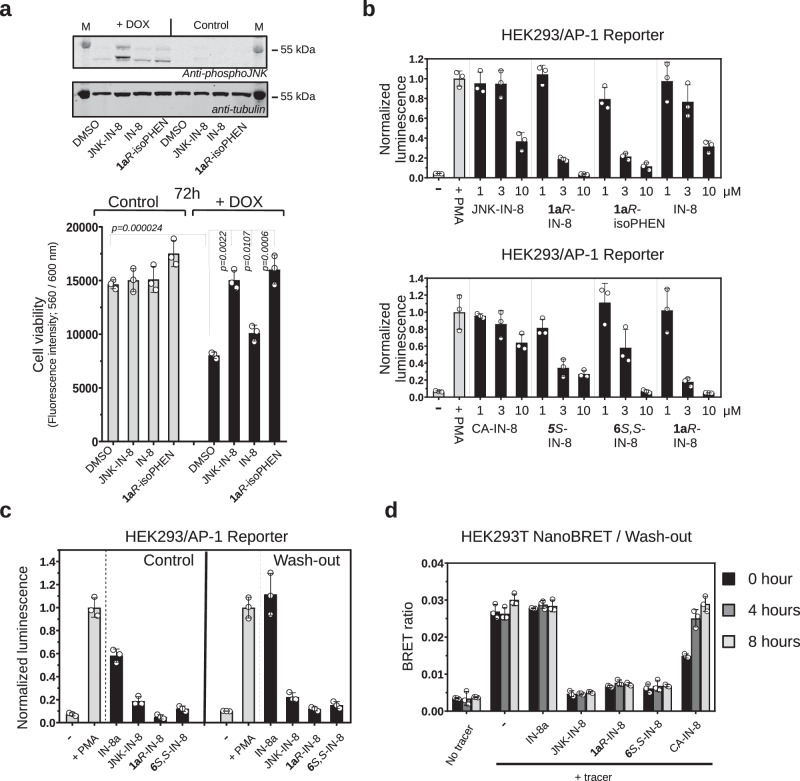
Fig. 4. Characterization of covalent JNK inhibitors in cell-based tests.
a Effect of inhibitors on JNK mediated cell death in SH-SY5Y MKK7 ACT cells. Endogenous JNK activation was initiated artificially by the addition of 2 μg/mL doxycycline (DOX) for 72 h in engineered SH-SY5Y neuroblastoma cell line (in which the expression of an active MLK3-MKK7 chimera is controlled via the DOX dependent Tet-ON system). The top panel shows the results of a phospho-JNK western blot confirming JNK activation upon doxycycline treatment ( + DOX; the two different bands on the phospho-JNK western blot correspond to different JNK isoforms). The panel below shows the results of the experiment with 1 μM JNK-IN-8 (irreversible), IN-8 (the ATP-pocket binding moiety without any warhead) or the reversible covalent 1aR-isoPHEN. (Data show the mean value and error bars show SD, n = 3, independent experiments; p-values were calculated based on two-sided, unpaired t-test.). b Effect of inhibitors on JNK mediated AP-1 transcription factor promoter activity. Reporter AP-1 – HEK293 Recombinant Cell Line was unstimulated (-) or stimulated with phorbol 12-myristate 13-acetate ( + PMA) and AP-1 promoter driven transcription of the luciferase reporter gene was monitored by measuring luminescence after 6 h. Inhibitors were co-administered with PMA and were used in 1, 3, or 10 μM concentrations. (Data show the mean and error bars show SD, n = 3, independent experiments.). c Results of wash-out experiments with noncovalent (IN-8a), irreversible covalent (JNK-IN-8) and reversible covalent (1aR-IN-8 or 6S,S-IN-8) inhibitors in the AP-1 promoter assay. Inhibitors (10 μM) were incubated with Reporter AP-1 – HEK293 cells for 4 h then were left untreated (-) or stimulated with phorbol 12-myristate 13-acetate ( + PMA). AP-1 promoter activity was monitored by luminescence measurements after 6 h. Wash-out samples were washed by PBS twice before adding fresh media with PMA (but without any inhibitor), while for control cells the media, in addition to PMA, contained the respective inhibitor in the same concentration as before the wash-out. (Data show the mean and error bars show SD, n = 3, independent experiments.). d Results of wash-out experiments monitoring long-term target engagement by NanoBRET. HEK293T cells were transiently transfected with a NanoLuc-JNK1 fusion expression plasmid and the binding of a fluorescent JNK ATP-pocket binding compound (tracer) was monitored in the absence or presence of different JNK inhibitors used in 10 μM concentration. The BRET ratio is low when there was no tracer added (No tracer), while the maximum BRET signal is expected upon addition of the tracer with no inhibitor added (-). Cells were preincubated with the inhibitors for 2 h and washed three times with media followed by the addition of fresh media supplemented with the tracer. The remaining amount of the respective JNK-inhibitor complex was monitored by the BRET signal right after of the wash-out (0 h) or 4 and 8 h later. (Data show the mean and error bars show SD, n = 3, independent experiments.) Source data are provided as a Source Data file.