Abstract
Cadmium (Cd) is an unessential and pervasive contaminant in agricultural soil, eventually affecting the food and instigating health issues. The implication of nanocomposites in agriculture attained significant attention to drive food security. Nanocomposites possess exceptional characteristics to stun the challenges of chemical fertilizers that can enhance plant yield and better nutrient bioavailability. Similarly, biochar has the ability to immobilize Cd in soil by reducing mobility and bioavailability. Rice husk biochar is produced at high temperature pyrolysis under anoxic conditions and a stable carbon-rich material is formed. To strive against this issue, rice plants were subjected to Cd (15, 20 mg kg− 1) stress and treated with alone/combined Ca + Mg (25 mg L− 1) nanocomposite and rice husk biochar. In our study, growth and yield traits showed the nurturing influence of Ca + Mg nanocomposite and biochar to improve rice defence mechanism by reducing Cd stress. Growth parameters root length 28%, shoot length 34%, root fresh weight 19%, shoot fresh weight 16%, root dry weight 9%, shoot dry weight 8%, number of tillers 32%, number of grains 20%, and spike length 17% were improved with combined application of Ca + Mg and biochar, with Cd (20 mg kg− 1), rivalled to alone biochar. Combined Ca + Mg and biochar application increased the SPAD 23%, total chlorophyll 26%, a 19%, b 18%, and carotenoids 15%, with Cd (20 mg kg− 1), rivalled to alone biochar. MDA 15%, H2O2 13%, and EL 10% were significantly regulated in shoots with combined Ca + Mg and biochar application with Cd (20 mg kg− 1) compared to alone biochar. POD 22%, SOD 17%, APX 18%, and CAT 9% were increased in shoots with combined Ca + Mg and biochar application with Cd (20 mg kg− 1) compared to alone biochar. Cd uptake in roots 13%, shoots 14%, and grains 21% were minimized under Cd (20 mg kg− 1) with combined Ca + Mg and B. pumilus application, compared to alone biochar. Subsequently, combined Ca + Mg and biochar application is a sustainable solution to boost crop production under Cd stress.
Keywords: Calcium, Magnesium, Nanocomposite, Rice, Biochar
Subject terms: Photosynthesis, Plant development, Plant immunity, Plant physiology, Plant stress responses, Secondary metabolism, Environmental sciences
Introduction
Cadmium (Cd) toxicity is a substantial environmental dominant factor in decreasing rice growth and yield1,2. Higher Cd toxicity and its persistence not only impact food safety but also cause health risks3–6. Cd abruptly uptake by the plants and translocated to shoots from roots. Cd toxicity pointedly abridged the height, weight, photosynthesis, chlorophyll contents and oxidant enzyme activities of rice7. Cd toxicity exerts a negative influence on agronomic traits reducing plants height, length of roots and shoots, fresh and dry weights of roots and shoots, SPAD values and antioxidant enzyme properties like SOD, POD, CAT and APX8. Cd contamination impacts wheat production by declining growth, nutrient imbalance, chlorophyll contents and antioxidant characteristics9–11. Additionally, Cd higher toxicity in rice producing regions in southern China has been increasing per annum and decreasing yield12. Therefore, there is a need for innovative, practical and sustainable stratagems to control the absorption of cadmium in edible crops.
Rice is an essential food that has been grown in numerous regions globally, providing indispensable food for more than half the inhabitants of the world. Roughly one-third of worldwide rice is cultivated in China. It is momentous that the domestic rice demand will increase by 20% till 2030 respective to population growth2. Considering the huge requirement, supplementing rice production compared to the chances of abiotic stress aspects, including soil Cd toxicity, instantly decreasing production. Reduction in grain yield, and higher Cd translocation in rice tissues subsequently a momentous involvement of dietetic Cd intake13. Cd semblance to zinc (Zn) causes rapid absorption, translocation, and bioaccumulation through cooperative modulation of Zn, dictating the decreased Cd uptake strategy14,15. In southern China, rice crop toxicity exceeds the admissible Cd and Pb levels (0.2 mg kg− 1, GB2726-2012) agreed with China National Food Safety Standards16. Cd may be mobilized into paddy fields via several mechanisms including irrigation water, atmospheric deposition, and agricultural practices together with the usage of chemical fertilizers and pesticides17. Henceforth, addressing soil Cd pollution is crucial for promising the imperishable use of arable land and secure food production18.
Calcium nanoparticles (Ca NPs) are used as nano fertilizers to give nutrients like calcium and make some bound minerals available through amendments to pH, organic matter, and cation exchange capacity19. Ca is an essential nutrient that is necessary for plant growth, photosynthesis, hormone production, cell walls, membranes, oxidative stress, chemical reactions, and the transportation of coupling messages responsible for extracellular signals and intracellular physiological responses, as well as the reduction of soil salinity20. It supports metabolic processes persuading absorption of supplementary vital minerals and stimulating cell elongation contributing to the consolidation of hormonal processes21. Mg2+, on the other hand, is widely distributed and functions as a cofactor for a variety of enzymes, including the core metal of green tissues’ chlorophyll molecules22. Plant roots absorb Ca2+ and Mg2+ from the soil and transport them upward to shoots via the xylem. Ca2+ is comparatively stationary once deposited, but Mg2+ is further mobile and can be recycled in plants via phloem. Several groups of ion channels and transporters supply Ca2+ and Mg2+ through plasma and intracellular membranes. Function proficiency of these passageways administers Ca2+ and Mg2+ feeding and assures suitable management of Ca2+ as a second messenger against diverse developmental and environmental signals to the cell and entire plant level23.
Similar to nanotechnology, biochar has attracted consideration for resolving Cd toxicity in soil for the long term. Additionally, several cases exhibited that biochar comprises innumerable nutrients (Mg, Na, K, P, and N), having incomparable advantages for growth and augmentation of crop production24. Biochar has the capability to alleviate metal toxicity, reduces uptake, and transportability by limiting metal phytotoxicity and translocation cultivated in contaminated soil25,26. Rice straw and bamboo derivative biochar application convinced the Cu, Zn, Pb and Cd immobilization in contaminated soil through declined heavy metals absorption in plants26. Additionally, biochar’s impact collectively with nano fertilizers to upgrade soil fertility and crop yield was well stated27–29. Little research work has been accomplished to inspect how this combination affects the uptake of Cd and the production of rice, the distinction of soil microorganisms along intricate association among microbe-plant-soil relationship and Cd alteration2. The role of NPs on plant species and heavy metal toxicity has been listed in Table 1. Therefore, there is an urgent need for a detailed outcome of biochar combined with Ca-Mg nanocomposite on Cd uptake in rice production. Among the recent remediation technologies, organic amendments are more cost-effective and intricate in the immobilization of heavy metals30. So, keeping in view the above literature and remediation technologies, we planned this study to examine the combined role of novel nanocomposite and rice husk biochar to enhance rice yield. No one examined the potential of Ca + Mg nanocomposite as a foliar application on plants. Till now, researchers only focused on single Ca and Mg nanoparticles or their combination.
Table 1.
Effects of nanocomposite on different heavy metals toxicity.
| NPs type | Synthesis | Dose applied | Toxicities | Crops | Effects | Reference |
|---|---|---|---|---|---|---|
| Calcium oxide | Chaoweii Nanotechnology company, Shanghai, China | – | Cd | Barley | Increased plants physiology, nutrients, and anti-oxidative enzymes and reduced Cd accumulation | 78 |
| Magnesium oxide | Green synthesis | 200 mg kg− 1 | As | Rice | Increased plants biomass, antioxidant enzyme activities and decreased ROS generation | 96 |
| Iron | Bought from Alfa Aesar co. | 0, 25, 50, and 100 mg kg− 1 | Cd and drought | Wheat | Decreased oxidative stress and increased wheat yield and Fe in grains | 97 |
| Magnesium oxide | Bought from Sigma Aldrich | 5 mmol L− 1 | Pb | Daucus carota | Detoxify the ROS and escalated the activity of SOD and CAT | 98 |
| Nano-ferrous sulfide | Hydrogel method | 1.5, 15, 30 gm− 2 soil | Cd | Water spinach | Increased soil nitrogen and organic matter and nutrients | 99 |
| Iron oxide | Bought from Pantian co. Shanghai | 50–200 mg L− 1 | Cd | Rice | Increased growth, reduced oxidative stress and bioaccumulation of Cd | 100 |
| Silicon oxide | Sol-gel method | 250 mg kg− 1 | Cr | Wheat | Boosted photosynthesis activity, antioxidant enzyme activity and abridged the ROS | 101 |
| Zinc and iron oxide | Zn (20–40 mgL− 1) Fe (10–20 mgL− 1) | As | Spinach | 102 |
Currently, the basic objectives of this study were to examine: (1) the combined effect of Ca + Mg nanocomposite and rice husk biochar on growth, photosynthesis attributes, yield, and Cd alleviation in rice; (2) the generation of reactive oxygen species (ROS) and secondary metabolites; (3) alone or combined Ca + Mg nanocomposite and rice husk biochar influence on uptake of Cd and nutrient profile. Hence, the existing study could propose an economically feasible substitute fertilizer that promotes sustainable agricultural crop yield with higher nutritional value.
Materials and methods
Soil collection and scrutiny
Soil (0–15 cm) was taken from the agriculture farms of the University of Agriculture, Faisalabad-Pakistan. After being air dried, the soil was sieved using a 2 mm sieve. The soil was sandy clay loam31, with electrical conductivity (EC) of 1.8dS m− 1 and pH (7.71) of the soil extract. Available Cd (0.07 mg kg− 1) by following the standard procedure of Amacher32. Walkley-Black protocol was followed to assess soil organic carbon, calcium carbonate, total nitrogen, available phosphorous, extractable potassium, and cation exchange capacity 3.18 g kg− 1, 3.3%, 0.086%, 6.2 mg kg− 1, 91 mg kg− 1, 10.3cmol(+) kg− 1, correspondingly. Similarly, the Zn, Mn, and Fe 5.2 mg kg− 1, 4.9 mg kg− 1, and 53.6 mg kg− 1 were scrutinized by undermentioned the calcimeter process33,34.
Preparation of rice husk biochar and Ca + Mg nanocomposites
Using pyrolysis, rice straw was used to make biochar in an oven set at 450 °C for two hours. Organic carbon 41.2%, pH 9.7, and EC 2.2dSm− 1 were noted after drying biochar at 105 °C for 24 h35. Synthesis and characterization of Ca + Mg nanocomposite is described in our previous study36.
Pot experiment
A pot experiment was carried out within natural environmental circumstances (day-night temperature, 39/32 ◦C, and humidity, 78 ± 4%) at the Botanical Garden of Government College University Faisalabad, Pakistan. The treatment plan is listed in Table 2. Pots were filled up with sifted soil (5 kg in each pot) spiked with Cd (0, 15 & 20 mg kg− 1) using cadmium chloride according to the treatment plan. A completely randomized design was used to conduct the experiment in triplicate. Rice seeds were submerged in water for 48 h and grown in sieved and washed sand. After 20 days of germination, the rice plants were shifted into pots containing 4 healthy plants. The recommended dose of NPK fertilizer was applied to avoid nutrient deficiency. The foliar application of Ca + Mg nanocomposite was applied after germination. A total of seven foliar applications were sprayed after one and a half weeks.
Table 2.
The details of treatments used in experiment.
| Treatments | Concentrations |
|---|---|
| T-1 | Control |
| T-2 | Cd 15 mg kg− 1 |
| T-3 | Cd 20 mg kg− 1 |
| T-4 | Biochar (2%) |
| T-5 | Biochar (2%) + Cd 15 mg kg− 1 |
| T-6 | Biochar (2%) + Cd 20 mg kg− 1 |
| T-7 | (Ca + Mg) nanocomposite 25mg L− 1 |
| T-8 | (Ca + Mg) nanocomposite 25mg L− 1+Cd 15 mg kg− 1 |
| T-9 | (Ca + Mg) nanocomposite 25mg L− 1+Cd 20 mg kg− 1 |
| T-10 | Biochar (2%) + Ca + Mg 25mg L− 1 |
| T-11 | Biochar (2%)+(Ca + Mg) nanocomposite 25mg L− 1+Cd 15 mg kg− 1 |
| T-12 | Biochar (2%)+(Ca + Mg) nanocomposite 25mg L− 1+Cd 20 mg kg− 1 |
Measurement of photosynthetic pigments and gas exchange parameters
SPAD (Soil plant analysis development) value was measured using an in-situ SPAD meter. Photosynthetic pigments such as chlorophyll a & b, total chlorophyll, and carotenoids were determined spectrophotometrically in fresh rice leaves37. Acetone 85% (v/v ratio) was used to extract the samples to assess photosynthetic pigments. Readings were noted on the spectrophotometer after extraction and centrifugation of samples. The gas exchange parameters (photosynthesis rate, transpiration rate, stomatal conductance, and water use efficiency) were measured during sunlight (12:00 a.m.) in leaves of rice plants using a portable CIRAS-3 (PP System, Amesbury, MA, USA).
Harvesting of plants
Plants were harvested and separated into different parts (shoots, roots, leaves, and grains) after 120 days of sowing. Growth characteristics were measured, including spike length, number of grains, fresh and dry weights of the roots and shoots (g), and lengths of the roots and shoots (cm). HCl (0.1%) and distilled water was used to wash roots to eliminate metals. Oven-dried (72 h at 80 °C) root, shoot, and grain samples were crumpled into tiny slices for further analysis.
Measurement of oxidant and antioxidant enzyme activities
Malondialdehyde (MDA) level was assessed with TBA (0.1%) method38,39. Electrolyte leakage determination was achieved in two steps. Initial EC was noted after incubating samples for 2 h at 32 °C. Second EC was noted for 20 min at 121 °C by following Dionisio-Sese and Tobita40. H2O2 activity was analyzed by the undermentioned process41. Following a 20-minute centrifugation, the samples were pulverized in phosphate buffer (PB 5mM and pH 6.5). After centrifugation, 20% sulfuric acid was added, and the mixture was centrifuged once more for 15 min. A spectrophotometer was then used to measure absorbance at 410 nm. Superoxide radical (O2·–) contents were measured by obtaining fresh leaf extract in hydroxylamine hydrochloride, titrated with naphthylamine (7 mM) and sulfanilamide (17 mM)42.
Phosphate buffer (PB 0.5, at pH 7.8) was used to crush the leaves and root samples for the determination of peroxidase (POD) and superoxide dismutase (SOD) contents43. Aebi44 approach was utilized to evaluate the contents of CAT, whereas the Nakano and Asada45 methodology was followed to estimate ascorbate peroxidase (APX).
Estimation of metabolites
Total soluble proteins (TSP) were measured by homogenizing fresh leaves (0.5 g) in potassium phosphate buffer (50 mM, pH 7.5) by following Bradford46. The fresh leaves (0.5 g) were crushed in a potassium phosphate buffer solution (50 mM, pH 7.5). Pyridine and acid ninhydrin were titrated with supernatant to measure total free amino acids (TFAA)47. Phenolics were determined by crushing fresh leaves (0.5 g) in acetone, centrifuged (10000 g, 10 min), and the supernatant was reacted with Folin and Ciocalteau’s phenol reagent48. Fresh leaves (0.5 g) were homogenized in a mixture of ethanol and water to determine the total soluble sugars (TSS), and the resulting extract was then treated with an anthrone reagent49.
Estimation of metal contents and micro-macronutrients
The procedure of Lwalaba et al.50 was adopted with slight modifications. Briefly, samples were digested on a hotplate at 140 ◦C using diacid HNO3: HClO4 (4 :1 v/v). Elements such as Ca, Mg, Mn, Zn, Fe, K, and Cd concentrations were determined using ICP-MS (iCAP RQ, Thermo Scientific).
Statistical analysis
Data analysis was carried out utilizing SPSS version 16.0 (SPSS, Chicago, IL). One-way analysis of variance (ANOVA) was conducted, following the Tuckey HSD test to describe substantial differences among means.
Results
Effect on growth parameters and yield
The consequence of the foliar application of nanocomposite and biochar is shown in Table 3 and overall effect of nanocomposite and biochar is diagrammed in Fig. 1. The results manifested that Cd 15/20 mg kg− 1 passivate the shoot and root length of rice 27/44% and19/38%, in contrast to the control treatment. While employment of 2% rice husk biochar ameliorated the shoot and root length of rice 23% and 22% in contrast to control treatments. Correspondingly, Ca + Mg nano composite augmented the shoot and root length of rice by 14% and 13% contrasted to control treatments. Although, the mutual application of composite nanoparticles and microbial inoculation momentous upsurges the rice growth. Combined Ca + Mg and rice husk biochar enhanced the shoot and root length 11% and 15% in comparison to the solo biochar application.
Table 3.
Growth parameters under different treatments.
| Treatments | RL | SL | RFW | SFW | RDW | SDW | NoT | NoG | SpL |
|---|---|---|---|---|---|---|---|---|---|
| Control | 9 ± 0.96bcd | 63 ± 3.06cde | 13 ± 0.60bc | 92 ± 4.72bcd | 1.2 ± 0.11bcd | 35 ± 1.98bcd | 8 ± 0.77bc | 27 ± 1.49cde | 11 ± 0.67 cd |
| Cd (15 mg kg− 1) | 7 ± 0.33def | 46 ± 3.54ghi | 10 ± 1.22de | 80 ± 5.49de | 1 ± 0.05de | 28 ± 2.39e | 5 ± 0.77de | 20 ± 1.29 g | 9 ± 1.05ef |
| Cd (20 mg kg− 1) | 6 ± 0.44f | 35 ± 2.57i | 9 ± 1.27e | 63 ± 5.49f | 0.8 ± 0.06e | 21 ± 2.59f | 3 ± 0.77e | 15 ± 1.71 h | 7 ± 0.79 g |
| Biochar | 11 ± 0.55ab | 77 ± 3.34ab | 16 ± 0.99a | 106 ± 5.5ab | 1.5 ± 0.08ab | 42 ± 2.37a | 11 ± 1.18ab | 32 ± 1.49ab | 14 ± 0.64ab |
| Cd (5 mg kg− 1) + Biochar | 9 ± 0.32cde | 60 ± 2.66ef | 13 ± 1.30bcd | 90 ± 6.73cde | 1.2 ± 0.12bcd | 34 ± 1.50bcd | 9 ± 0.44bc | 26 ± 1.49de | 11 ± 0.55cde |
| Cd (10 mg kg− 1) + Biochar | 7 ± 0.52ef | 45 ± 1.79hi | 10 ± 1.36cde | 75 ± 5.31ef | 1.1 ± 0.11de | 29 ± 2.02de | 6 ± 1.18 cd | 22 ± 1.53 fg | 9 ± 0.43def |
| (Ca + Mg) nanocomposite (25 mg L− 1) | 11 ± 0.97bc | 71 ± 3.27bc | 15 ± 0.71ab | 100 ± 5.40abc | 1.4 ± 0.10abc | 37 ± 2.12abc | 10 ± 0.77ab | 30 ± 1.69bcd | 13 ± 0.28bc |
| Cd (15 mg kg− 1) + (Ca + Mg) nanocomposite (25 mg L− 1) | 9 ± 1cde | 56 ± 1.69efg | 12 ± 1.27bcd | 88 ± 5.31cde | 1.2 ± 0.06 cd | 32 ± 2.59bcde | 8 ± 0.44bc | 25 ± 1.27ef | 11 ± 1.08cdef |
| Cd (20 mg kg− 1) +(Ca + Mg) nanocomposite (25 mg L− 1) | 7 ± 0.71ef | 44 ± 2.88fgh | 10 ± 0.71de | 74 ± 5.40ef | 1 ± 0.06de | 27 ± 1.59ef | 5 ± 0.77de | 20 ± 1.27 g | 9 ± 0.59 fg |
| Biochar + (Ca + Mg) nanocomposite (25 mg L− 1) | 13 ± 0.82a | 85 ± 2.96a | 17 ± 0.95a | 113 ± 4.81a | 1.6 ± 0.11a | 43 ± 2.57a | 12 ± 0.77a | 35 ± 1.07a | 15 ± 0.83a |
| Cd (15 mg kg− 1) + Biochar + (Ca + Mg) nanocomposite (25 mg L− 1) | 11 ± 0.75bc | 70 ± 2.90bcd | 15 ± 0.58ab | 100 ± 5.36abc | 1.4 ± 0.09abc | 37 ± 1.70ab | 10 ± 1.18ab | 31 ± 1.07bc | 13 ± 0.79bc |
| Cd (20 mg kg− 1) + Biochar + (Ca + Mg) nanocomposite (25 mg L− 1) | 9 ± 0.94cde | 60 ± 3.60de | 12 ± 0.67bcd | 87 ± 5.27cde | 1.2 ± 0.07 cd | 31 ± 2.15cde | 8 ± 0.77bc | 26 ± 1.53de | 11 ± 0.45cde |
Fig. 1.
Overall effect of Ca + Mg nanocomposite and biochar on rice.
The contamination level of Cd 15/20 mg kg− 1 attenuates shoot fresh and dry weight 13/32% and 20/39% over the control. Whereas, the application of 2% rice husk biochar enunciates upsurge the shoot fresh and dry weight of rice 15% and 23% comparing with the control. Ca + Mg nano composite augments the shoot fresh weight and dry weight of rice 8% and 7% as compared to without stress treatments. Besides this, the joint application of microbes and nanocomposite improved the shoots fresh and dry weights. Application of rice husk biochar and nanocomposite (Ca + Mg) collectively augmented the shoot fresh weight also dry weight of rice 7% and 2% concerning the alone application of biochar.
The results manifested that contamination of Cd 15/20 mg kg− 1 diminished the root fresh and dry weight of rice 22/36% and 19/35% compared to the control. The application of 2% rice husk biochar escalates the root fresh weight as well as the dry weight of rice 22% and 17% in comparison to control treatments. Likewise, the foliar employment of composite (Ca + Mg) nanoparticles augmented the root fresh and dry weight of rice 13% and 10% in contrast to no stress. Whereas the combined application of rice husk biochar and nanocomposite (Ca + Mg) nanoparticles amplified the root fresh weight also dry weight of rice 6% and 7% as compared to the alone application of biochar.
The current study revealed that Cd 15/20 mg kg− 1 suppressed the No. of tillers, grains, and spike length of rice 36/63%, 24/42%, and 21/41% respectively over the control. Moreover, the application of 2% rice husk biochar notably augmented the No. of tillers, grains, as well as spike length of rice 24%, 19%, and 22% respectively in contrast to control treatments. While Ca + Mg nanocomposite enhanced the No. of tillers, grains, together with spike length of rice 18%, 10%, and 13% respectively in contrast to control. Henceforth combined application of composite (Ca + Mg) nanoparticles and rice husk biochar improved the No. of tillers, grains, and spike length in rice. Combined employment ameliorated the No. of tillers, grains, and spike length of rice 17%, 9%, and 12% respectively in comparison to the application of biochar.
Effect on SPAD values
The statistical analysis showed that the SPAD meter values under Cd pressure decreased significantly, as shown (Table 4). The results exhibited Cd 15/20 mg kg− 1 decreased the SPAD meter values of rice 19% and 39% for control treatment. Rather than these, the application of 2% rice husk biochar increased the SPAD meter values of rice 12% as compared to control treatments. While the Ca + Mg nanocomposite increased the SPAD meter values of rice 8% in contrast to the control. Ca + Mg nanocomposite and rice husk biochar significantly enhanced the SPAD meter values. The results showed that combined application increased the SPAD meter values of rice 7% as compared to the alone application of biochar.
Table 4.
Photosynthetic pigments under different treatments.
| Treatments | SPAD | Chl a | Chl b | Total chl | Carotenoids | Pn | Tr | WUE | SC |
|---|---|---|---|---|---|---|---|---|---|
| Control | 42 ± 2.50bc | 2.75 ± 0.10 cd | 1.52 ± 0.10 cd | 4.46 ± 0.30cde | 1.14 ± 0.09 cd | 10 ± 0.40bc | 3 ± 0.14 cd | 6 ± 0.20 cd | 2 ± 0.09c |
| Cd (15 mg kg− 1) | 34 ± 3.04d | 2.18 ± 0.10f | 1.23 ± 0.09ef | 3.45 ± 0.25 fg | 0.76 ± 0.09fgh | 8 ± 0.50de | 2 ± 0.10f | 5 ± 0.35 g | 1 ± 0.04de |
| Cd (20 mg kg− 1) | 25 ± 2.98e | 1.74 ± 0.15 g | 0.76 ± 0.10 g | 2.66 ± 0.30 g | 0.5 ± 0.05 h | 6 ± 0.50f | 2 ± 0.20 g | 4 ± 0.40 h | 1 ± 0.09e |
| Biochar | 47 ± 2.90ab | 3.23 ± 0.10ab | 1.90 ± 0.10b | 5.32 ± 0.30b | 1.43 ± 0.09ab | 11 ± 0.57b | 3 ± 0.10ab | 8 ± 0.40ab | 2 ± 0.09ab |
| Cd (15 mg kg− 1) + Biochar | 41 ± 2.92 cd | 2.66 ± 0.10de | 1.54 ± 0.11de | 4.25 ± 0.33def | 1.07 ± 0.05cde | 9 ± 0.50 cd | 2 ± 0.13bde | 6 ± 0.33cde | 2 ± 0.09 cd |
| Cd (20 mg kg− 1) + Biochar | 31 ± 3.60de | 2.21 ± 0.14f | 1.23 ± 0.09ef | 3.67 ± 0.34ef | 0.85 ± 0.09efg | 7 ± 0.51e | 2 ± 0.15ef | 5 ± 0.50efg | 1 ± 0.06e |
| (Ca + Mg) nanocomposite (25 mg L− 1) | 45 ± 1.99abc | 3 ± 0.10bc | 1.77 ± 0.14bc | 5.13 ± 0.30bc | 1.28 ± 0.09bc | 11 ± 0.60b | 3 ± 0.10bc | 7 ± 0.36bcd | 2 ± 0.11b |
| Cd (15 mg kg− 1) + (Ca + Mg) nanocomposite (25 mg L− 1) | 38 ± 2.37 cd | 2.56 ± 0.18d | 1.45 ± 0.06de | 4.10 ± 0.27def | 0.85 ± 0.09efg | 9 ± 0.60 cd | 2 ± 0.18bd | 6 ± 0.45def | 2 ± 0.10c |
| Cd (20 mg kg− 1) +(Ca + Mg) nanocomposite (25 mg L− 1) | 29 ± 2.85de | 2.18 ± 0.11def | 1.15 ± 0.07f | 3.61 ± 0.30f | 0.66 ± 0.09gh | 7 ± 0.60ef | 2 ± 0.18ef | 5 ± 0.41 fg | 1 ± 0.0.06de |
| Biochar + (Ca + Mg) nanocomposite (25 mg L− 1) | 50 ± 2.25a | 3.51 ± 0.10a | 2.18 ± 0.10a | 6.27 ± 0.30a | 1.66 ± 0.09a | 13 ± 0.56a | 4 ± 0.10a | 8 ± 0.36a | 3 ± 0.04a |
| Cd (15 mg kg− 1) + Biochar + (Ca + Mg) nanocomposite (25 mg L− 1) | 44 ± 2.26abc | 3 ± 0.10bc | 1.75 ± 0.06 cd | 5.25 ± 0.29bc | 1.23 ± 0.09bcd | 11 ± 0.60b | 3 ± 0.11bc | 7 ± 0.40bc | 2 ± 0.08b |
| Cd (20 mg kg− 1) + Biochar + (Ca + Mg) nanocomposite (25 mg L− 1) | 38 ± 2.74 cd | 2.64 ± 0.12d | 1.46 ± 0.07de | 4.63 ± 0.16bcd | 0.98 ± 0.05def | 9 ± 0.60 cd | 2 ± 0.10d | 6 ± 0.41cde | 2 ± 0.16c |
Effect on chlorophyll contents
Chlorophyll content a, b, total chlorophyll, and carotenoid were suppressed under Cd stress, as shown (Table 4). Briefly, Cd 15/20 mg kg− 1 declined chlorophyll a, b, total chlorophyll, and carotenoids 21/37%, 19/50%, 22/40%, and 33/56% correspondingly as contrasted with control treatments. While the employment of 2% rice husk biochar enhanced chlorophyll a, b, total chlorophyll, and carotenoids 17%, 25%, 19%, and 26% respectively as compared to control treatments. Similarly, Ca + Mg nano composite boosted chlorophyll a, b, total chlorophyll, and carotenoids of rice 10%, 17%, 15%, and 12% respectively in contrast to without toxicity. Although combined rice husk biochar and Ca + Mg nano composite significantly improved the chlorophyll a, b, total chlorophyll and carotenoid 9%, 15%, 18%, and 16% respectively in contrast to alone biochar application.
Effect on gas exchange characteristics
The statistical analysis showed that Cd contamination significantly decreased the photosynthesis rate (Pn), transpiration rate (Tr), water use efficiency (WUE), and stomatal conductance of rice, as depicted (Table 4). The results showed that Cd contamination of 15/20 mg kg− 1 decreased the Pn, Tr, WUE, and stomatal conductance of rice 20/44%, 23/40%, 22/43%, and 25/40% respectively over the control. While the application of 2% rice husk biochar increased the Pn, Tr, WUE, and stomatal conductance of rice 12%, 24%, 19%, and 26% respectively in contrast to the control. Besides this, Ca + Mg nanocomposite increased Pn, Tr, WUE, and stomatal conductance 11%, 13%, 10%, and 18% respectively concerning without stress. Similarly, microbial inoculation and Ca + Mg nano composite significantly increased the Pn, Tr, WUE, and stomatal conductance of rice. The results showed that the combined rice husk biochar and Ca + Mg nanocomposite increased the Pn, Tr, WUE, and stomatal conductance of rice 16%, 9%, 11%, and 12% respectively in contrast to the alone application of biochar.
Effect on EL, MDA, H2O2 and O2·
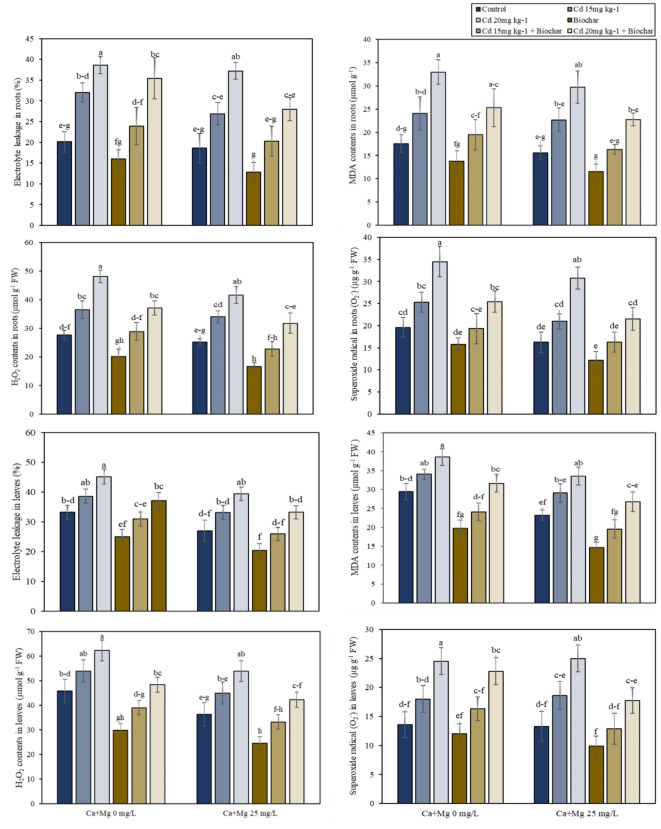
According to the results electrolyte leakage (EL), malondialdehyde (MDA), hydrogen peroxide (H2O2), and superoxide radical (O2·–) values in roots and shoots of rice significantly increased with Cd contamination (Fig. 2a). The results showed that the Cd 15/20 mgkg−1 increased the EL, MDA, H2O2, and O2·– values in roots of rice 58/91%, 37/87%, 32/74%, and 29/76% respectively over the control. Nevertheless, the application of 2% rice husk biochar decreased the EL, MDA, H2O2, and O2·– values in the roots of rice 21%, 22%, 27%, and 20% respectively as compared to control treatments. While foliar application of nanocomposite (Ca + Mg) nanoparticles decreased the values of EL, MDA, H2O2, and O2·– of rice 8%, 11%, 9%, and 17% respectively concerning control treatments. Similarly, the combined application of rice husk biochar and foliar application of nanocomposite (Ca + Mg) nanoparticles decreased the EL, MDA, H2O2, and O2·– values in roots of rice 20%, 16%, 18%, and 22% as compared to the alone application of biochar.
Fig. 2.
(a) Alone and combined effect of Ca + Mg nanocomposite (0, 25 mg L− 1) and rice husk biochar on oxidative stress markers EL, MDA, H2O2 in roots and leaves. Showing standard deviation at p ≤ 0.05 level with mean of three replications. (b) Alone and combined effect of Ca + Mg nanocomposite (0, 25 mg L− 1) and rice husk biochar on enzymatic antioxidants POD, SOD, APX and CAT in rice roots and leaves. Showing standard deviation at p ≤ 0.05 level with mean of three replications.
According to the results electrolyte leakage (EL), malonaldehyde (MDA), hydrogen peroxide (H2O2), and superoxide radical (O2·–) values significantly increased with Cd contamination. The results showed that the Cd 15/20 mg kg− 1 increased the EL, MDA, H2O2, and O2·– values in shoots of rice 16/36%, 15/31%, 18/36%, and 32/80% respectively over the control. Nevertheless, the application of 2% rice husk biochar decreased the EL, MDA, H2O2, and O2·– values in shoots of rice 25%, 33%, 35%, and 12% respectively in comparison to those without toxicity treatments. While Ca + Mg nanocomposite decreased the values of EL, MDA, H2O2, and O2·– in shoots of rice 19%, 21%, 20%, and 2% respectively concerning no stress treatments. Similarly, the combined implementation of rice husk biochar and foliar application of Ca + Mg nanocomposite decreased the EL, MDA, H2O2, and O2·– values in shoots of rice 18%, 26%, 18%, and 17% as compared to the alone application of biochar (Fig. 2a).
Effect on antioxidant enzyme activities
The results showed that Cd contamination decreased the POD, SOD, APX, and CAT in the roots and shoots of rice (Fig. 2b). The results showed that Cd contamination decreased the antioxidants enzyme activities including peroxidase (POD), superoxidase (SOD), ascorbate peroxidase (APX), and catalase (CAT) in the roots of rice. The results showed that Cd 15/20 mg kg− 1 decreased the values of POD, SOD, APX, and CAT in roots of rice 30/55%, 25/49%, 14/38%, and 21/47% respectively in comparison with the control treatment. Rather than this, the utilization of 2% rice husk biochar increased the POD, SOD, APX, and CAT values in roots of rice 22%, 33%, 14%, and 24% respectively as compared to the control treatments. While the foliar application of nanocomposite (Ca + Mg) nanoparticles increased the POD, SOD, APX, and CAT values in roots of rice 14%, 15%, 8%, and 14% respectively concerning control treatments. Similarly, the collective application of rice husk biochar and nanocomposite (Ca + Mg) nanoparticles enhanced the POD, SOD, APX, and CAT values in roots of rice 12%, 16%, 13%, and 28% as compared to the alone application of biochar.
The results showed that Cd 15/20 mg kg− 1 decreased the values of POD, SOD, APX, and CAT in shoots of rice 26/41%, 27/44%, 15/31%, and 18/36% respectively as contrasted with the control treatment. Rather than this the usage of 2% rice husk biochar increased the POD, SOD, APX, and CAT values of rice 14%, 14%, 13%, and 12% respectively as compared to the control treatments. While the foliar application of nanocomposite (Ca + Mg) nanoparticles increased the POD, SOD, APX, and CAT values in shoots of rice 7%, 9%, 10%, and 10% respectively concerning no stress treatments. Similarly, the combined application of rice husk biochar and nanocomposite (Ca + Mg) nanoparticles enhanced the POD, SOD, APX, and CAT values in shoots of rice 11%, 16%, 9%, and 16% as compared to the alone application of biochar (Fig. 2b).
Effect on metabolites
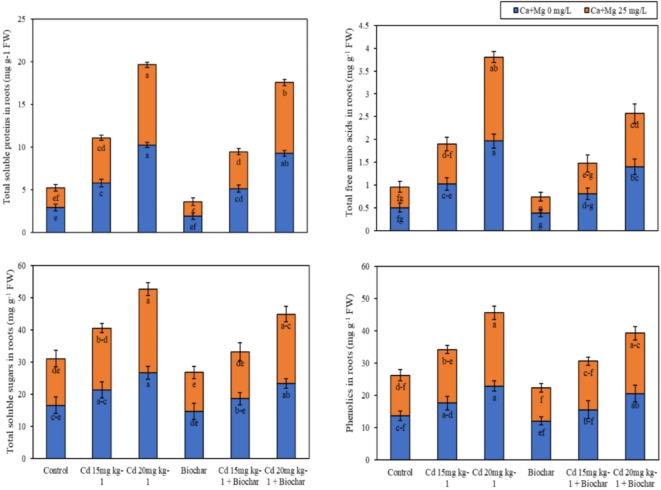
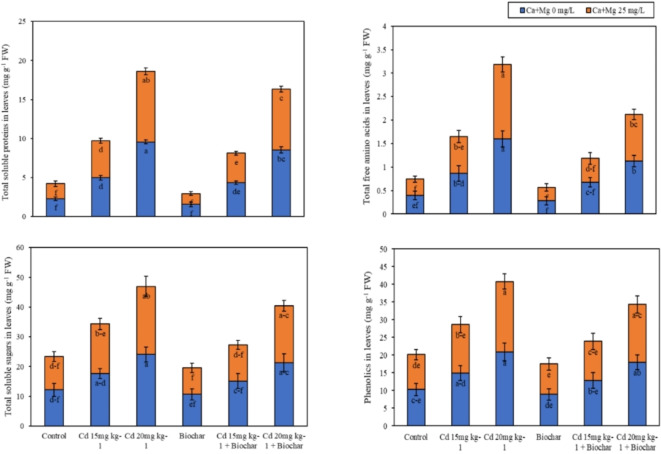
Foliar application of (Ca + Mg) nanocomposite and rice husk biochar showed a slight (p ≤ 0.05) effect on metabolites in leaves and roots such as total soluble proteins (TSP), total free amino acids (TFAA), total soluble sugars (TSS), and phenolics under Cd contamination. According to the results, Cd 15/20 mg kg− 1 significantly increased the TSP, TFAA, and TSS values in the roots of rice over the control (Fig. 3a). Nevertheless, the application of 2% rice husk biochar decreased the TSP, TFAA, TSS, and phenolics values in roots of rice 35%, 24%, 11%, and 11% respectively as contrary to control treatments. While Ca + Mg nanocomposite decreased the values of TSP, TFAA, TSS, and phenolics of rice 22%, 10%, 12%, and 8% respectively concerning control treatments. Similarly, the joint employment of rice husk biochar and foliar application of nanocomposite (Ca + Mg) nanoparticles decreased the TSP, TFAA, TSS, and phenolics values in roots of rice 11%, 6%, 18%, and 16% as contrasted to the alone implementation of biochar.
Fig. 3.
(a) Alone and combined effect of Ca + Mg nanocomposite (0, 25 mg L− 1) and rice husk biochar on total soluble proteins, total free amino acids, total soluble sugars and phenolics in roots. Showing standard deviation at p ≤ 0.05 level with mean of three replications. (b) Alone and combined effect of Ca + Mg nanocomposite (0, 25 mg L− 1) and rice husk biochar on total soluble proteins, total free amino acids, total soluble sugars and phenolics in leaves. Showing standard deviation at p ≤ 0.05 level with mean of three replications.
The results showed that the Cd 15/20 mg kg− 1 significantly increased the TSP, TFAA, and TSS values in the leaves of rice over the control (Fig. 3b). Nevertheless, the application of 2% rice husk biochar decreased the TSP, TFAA, TSS, and phenolics values in leaves of rice 32%, 29%, 13%, and 14% respectively as contrary to control treatments. While Ca + Mg nanocomposite decreased the values of TSP, TFAA, TSS, and phenolics of rice 16%, 12%, 8%, and 4% respectively concerning control treatments. Similarly, the combined application of rice husk biochar and foliar application of nanocomposite (Ca + Mg) nanoparticles decreased the TSP, TFAA, TSS, and phenolics values in leaves of rice 11%, 1%, 16%, and 3% as compared to the alone application of biochar.
Effect on macro and micronutrients
The results showed that Cd contamination decreased the macro and micronutrients including Zn, Fe, Mg, Mn, K, and Ca in shoots and grains of rice (Table 5). The outcomes exhibited that Cd 15/20 mg kg− 1 lessened the values of Zn, Fe, Mg, Mn, K, and Ca in shoots of rice 36/69%, 45/86%, 22/39%, 39/73%, 19/35%, and 13/24% respectively as contrary to the control treatment. Rather than this, the employment of 2% rice husk biochar amplified the Zn, Fe, Mg, Mn, K, and Ca values of rice 28%, 35%, 27%, 19%, 27%, and 3% respectively as contrary to no stress. Whereas the Ca + Mg nanocomposite augmented the Zn, Fe, Mg, Mn, K, and Ca values in shoots of rice 16%, 19%, 32%, 7%, 32%, and 17% respectively concerning control treatments. Similarly, the mutual submission of rice husk biochar and nanocomposite (Ca + Mg) nanoparticles boosted the Zn, Fe, Mg, Mn, K, and Ca values in shoots of rice 15%, 18%, 12%, 15%, 10%, and 26% as compared to the alone application of biochar.
Table 5.
Macro and micronutrient contents in shoots and grains under different treatments.
| Treatments | Shoots | Grains | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Micronutrients mg kg− 1 DW | Macronutrients mg kg− 1 DW | Micronutrients mg kg− 1 DW | Macronutrients mg kg− 1 DW | |||||||||
| Zn | Fe | Mn | Ca | Mg | K | Zn | Fe | Mn | Ca | Mg | K | |
| Control | 46 ± 3.89e | 36.23 ± 2.06d | 47.26 ± 2.95 cd | 215.17 ± 10.75 cd | 2035.6 ± 156.57f | 2126.8 ± 60.93df | 23.33 ± 0.85 cd | 18.98 ± 0.57ce | 24.73 ± 0.75bd | 112.55 ± 3.80ad | 1094.7 ± 76.25ce | 1206.6 ± 148.09bc |
| Cd (15 mg kg− 1) | 29.40 ± 2.36 g | 19.73 ± 3.62f | 8.80 ± 2.57f | 186.63 ± 14.40e | 1579 ± 176.72 h | 1714.3 ± 196.85 fg | 16.88 ± 2.09de | 12.11 ± 2.80gh | 16.26 ± 0.47ef | 96.69 ± 4.60 cd | 830.47 ± 101.39de | 756.31 ± 96.46ef |
| Cd (20 mg kg− 1) | 14.33 ± 1.80i | 4.96 ± 1.32i | 12.90 ± 1.55 h | 162.70 ± 7.96f | 1246.9 ± 78.66i | 1381.2 ± 187.08 g | 8.28 ± 0.41f | 4.33 ± 0.92i | 8.64 ± 1.52 g | 86.28 ± 13.98d | 441.59 ± 40.30f | 443.47 ± 24.41 g |
| Biochar | 58.90 ± 2.12b | 48.90 ± 1.68b | 56.53 ± 4.20b | 221.63 ± 25.45 cd | 2579.9 ± 149.44bc | 2696.9 ± 202.51ac | 30.43 ± 4.10ab | 25.83 ± 1.69b | 28.65 ± 1.60ab | 121.76 ± 3.68ac | 1334.7 ± 111.96ac | 1426.9 ± 161.17ab |
| Cd (15 mg kg− 1) + Biochar | 49.40 ± 4.90de | 38.80 ± 4.33de | 46.43 ± 3.85 cd | 210.57 ± 7.29d | 2261.1 ± 256.04e | 2388.9 ± 193.77be | 25.79 ± 3.04bc | 19.95 ± 1.54ce | 23.84 ± 2.72bd | 110.42 ± 4.06ad | 1100.9 ± 80.83 cd | 1022.6 ± 89.39ce |
| Cd (20 mg kg− 1) + Biochar | 39 ± 1.76f | 29 ± 1.76f | 39.43 ± 3.05e | 187.10 ± 8.86e | 1782.8 ± 156.75 g | 1924.3 ± 153.62eg | 21.03 ± 2.76 cd | 13.65 ± 2.03 fg | 16.08 ± 2.09ef | 98.36 ± 7.42 cd | 803.18 ± 14.44e | 714.21 ± 81.42 fg |
| (Ca + Mg) nanocomposite (25 mg kg− 1) | 53.10 ± 3.66 cd | 43.10 ± 1.74 cd | 50.56 ± 4.75c | 254.27 ± 15.84b | 2690.7 ± 248.68b | 2807.8 ± 285.50ab | 25.09 ± 4.50bc | 22.68 ± 1.36bd | 27.70 ± 2.52bc | 128.72 ± 11.81ab | 1472.2 ± 140.19ab | 1329.7 ± 78.63ab |
| Cd (15 mg kg− 1) + (Ca + Mg) nanocomposite (25 mg kg− 1) | 38.50 ± 1.95f | 28.50 ± 1.95f | 35.86 ± 3.95e | 235.33 ± 9.06bc | 2343 ± 68.70de | 2465.8 ± 146.99ae | 18.10 ± 0.43de | 15.06 ± 1.86eg | 20.86 ± 3.53de | 117.48 ± 11.87ac | 1217.9 ± 142.47bc | 864.96 ± 162.78ef |
| Cd (20 mg kg− 1) + (Ca + Mg) nanocomposite (25 mg kg− 1) | 23.30 ± 1.70 h | 13.30 ± 1.70 h | 20.53 ± 2.91 g | 214.23 ± 14.80 cd | 1937.3 ± 78.66 fg | 2069.8 ± 43.84df | 13 ± 1.30ef | 8.40 ± 0.69hi | 13.15 ± 1.78 fg | 106.83 ± 13.60bd | 842.56 ± 89.46de | 645.62 ± 32.80 fg |
| Biochar + (Ca + Mg) nanocomposite (25 mg kg− 1) | 67.90 ± 2.20a | 57.90 ± 6.20a | 65.23 ± 2.31a | 277.23 ± 7.01a | 2892.1 ± 242.96a | 2974.6 ± 140.32a | 34.33 ± 1.50a | 31.16 ± 1.87a | 34.49 ± 2.30a | 137.72 ± 11.03a | 1617.2 ± 146.01a | 1613.8 ± 42.43a |
| Cd (15 mg kg− 1) + Biochar + (Ca + Mg) nanocomposite (25 mg kg− 1) | 58.06 ± 2.11bc | 48.06 ± 2.11bc | 55.73 ± 1.33b | 257.23 ± 6.62ab | 2476.2 ± 80.78 cd | 2611.2 ± 225.63ad | 27.70 ± 0.65ac | 23.02 ± 1.70bc | 28.60 ± 1.64ab | 128.10 ± 12.74ab | 1213.7 ± 112.91bc | 1192 ± 20.51bd |
| Cd (20 mg kg− 1) + Biochar + (Ca + Mg) nanocomposite (25 mg kg− 1) | 46.7 ± 1.90e | 36.73 ± 1.95e | 44.10 ± 4.20d | 227.93 ± 10.14 cd | 2050.2 ± 149.95f | 2201.7 ± 246.70cf | 22.28 ± 1.66 cd | 17.90 ± 1.90df | 21.67 ± 1.88ce | 113.26 ± 11.73ad | 864.49 ± 26.17de | 894.94 ± 18.11df |
The results showed that Cd 15/20 mg kg− 1 attenuates the values of Zn, Fe, Mg, Mn, K, and Ca in grains of rice 28/65%, 36/77%, 24/60%, 34/765%, 37/63%, and 9/34% respectively as collated to the control treatment. Rather than this, the usage of 2% rice husk biochar upsurges the Zn, Fe, Mg, Mn, K, and Ca values of rice 30%, 36%, 22%, 16%, 18%, and 12% respectively as contrary to the without stress. While Ca + Mg nanocomposite aggrandized the Zn, Fe, Mg, Mn, K, and Ca values in grains of rice 8%, 20%, 34%, 12%, 10%, and 6% respectively concerning control treatments. Similarly, the collective usage of rice husk biochar and nanocomposite (Ca + Mg) nanoparticles enhanced the Zn, Fe, Mg, Mn, K, and Ca values in grains of rice 13%, 20%, 21%, 20%, 13%, and 3% as contrasted to the alone usage of biochar.
Effect on metal accumulation
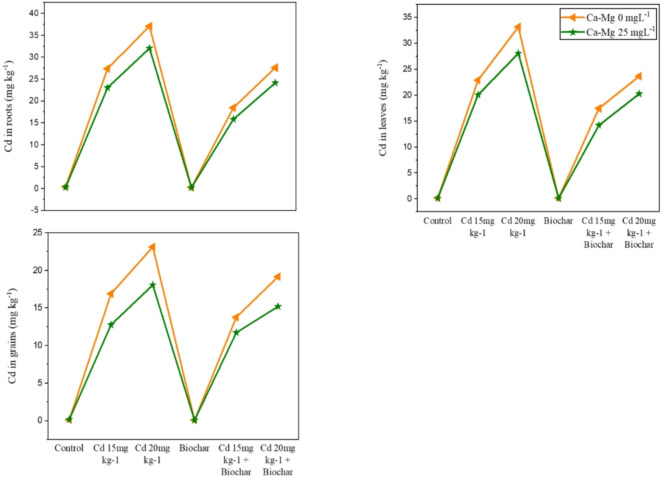
The statistical analysis showed that the soil spiked with Cd 15/20 mg kg− 1 augmented the Cd level in roots, shoots, as well as grains of rice significantly over the standard (Fig. 4). The application of 2% rice husk biochar decreased the Cd contamination in roots, shoots, and grains of rice 38%, 17%, and 20% respectively contrary to the control. Besides this, Ca + Mg nanocomposite decreased the Cd contamination of rice 30%, 33%, and 40% respectively concerning without toxicity. Similarly, the collective employment of rice husk biochar and foliar application of nanocomposite (Ca + Mg) nanoparticles decreased the Cd contamination in roots, shoots, and grains of rice 13%, 40%, and 50% as compared to the alone application of biochar.
Fig. 4.
Alone and combined effect of Ca + Mg nanocomposite (0, 25 mg L− 1) and rice husk biochar on Cd uptake and accumulation in roots, leaves and grains of rice. Showing standard deviation at p ≤ 0.05 level with mean of three replications.
Discussion
The present study was planned to examine the combined role of novel Ca + Mg nanocomposite and rice husk biochar to enhance rice growth and yield. The potential of Ca + Mg nanocomposite as a foliar application on plants was not investigated alone or combined with biochar till now. Researchers only focused on single Ca and Mg nanoparticles or their combination. Abundant studies manifested that the application of Ca and Mg nanoparticles certainly affects the plants growth and antioxidant defence system by lowering Cd stress51–53. Application of acidified rice husk biochar significant increment in rice growth parameters and reduced Cd toxicity54. There is a still research gap regarding the combined application of Ca-Mg nanocomposite and rice husk biochar on rice under Cd toxicity. The existing investigation has depicted the worthwhile effects of Ca-Mg nanocomposite and rice husk biochar on rice growth, photosynthetic pigments, antioxidant system, metabolites and nutrient profile under Cd stress.
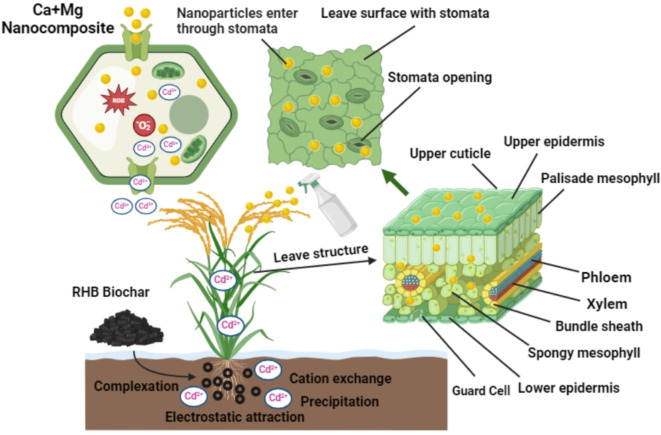
Ca regulates a range of activities within the cell, including cell division and elongation, cytoplasmic streaming, photomorphogenesis and plant defense conversely environmental stresses55,56. It is well-known that Ca roles as the central node in the inclusively signaling web and has an optimistic part in toxicity resistance57. Ca invigorates calmodulin-like proteins that interrelate with Ca2+ ions. Altering their configuration in response to Ca-binding, calmodulin proteins control innumerable mechanisms, including ion transport, gene regulation, cell motility, growth, proliferation, apoptosis and stress tolerance58,59. Magnesium (Mg) is considered indispensable constituent for plant development as it modulates several physiological and biochemical procedures and acts a crucial part in protecting plants beneath abiotic stress matrices60,61. Mg being a phloem mobilize element can transfer smoothly to the active developing parts of the plant or where it is compulsory for example chlorophyll generation, activation of enzyme mandatory for protein biosynthesis, and phloem exportation of photosynthates, to promise appropriate vegetative and reproductive development62. The proposed mechanism of Ca + Mg for the mitigation of Cd toxicity is shown in Fig. 5.
Fig. 5.
The proposed mechanism of Ca + Mg nanocomposite for the mitigation of Cd toxicity.
Biochar can diminution metal accumulation through plants via frequent mechanisms as well as mitigate metal movement in the soil, by altering the soil characteristics and the outcomes rely upon the category of biochar, kind of soil, and crops63. The extensively asserted advantages of biochar contain carbon (C) sequestration; upsurged soil water holding capacity and nutrient retention, boosted rhizospheric processes, organic carbon and escalated crop yield64–68. The capacity of biochar to attach metals is probably recognized owed to the interchange of ions among surface protons and metallic cations69. The negative surface charge on biochar allows it to adsorb Cd ions via electrostatic interactions, which helps in ion exchange by succeeding with other cations for binding sites. It alters the soil pH and affects the Cd precipitation and organic matter, and functional groups form complexes with Cd ions, eventually diminishing Cd availability for plant uptake70.
Currently, the probable impact of soil Cd toxicity on innocuous farming and the yield of crops has accomplished significant consideration. Prior research71, informed that the biochar in collaboration with ZnO NPs foliar spray was probably utilized to cultivate rice seedlings, particularly in zones where Cd absorption is increasing, and Zn deficit is greater. The collective treatment of nanocomposite, rice straw biochar, and cow manure biochar impacts sunflower oil quality and production along with diminishing the harmful influences of HMs72. In current research work, noticeable impacts of the co-application of foliar (Ca + Mg) nanocomposite and rice husk biochar on rice growth parameters, chlorophyll content, antioxidant enzyme activities, yield and Cd toxicity alleviation contrasted with single biochar and nanoparticles were detected.
The results of this study disclosed that the Cd contamination declined the values of growth parameters including root and shoot lengths, root and shoot fresh and dry weights, number of tillers, spike length and number of grains. A similar outcome was found in a previous study which reported that the Cd contamination lessened the values of growth parameters in rice plants11. Cd contamination decreased the values of growth parameters like root and shoot length, root and shoot fresh weight and dry weight, number of tillers, spike length, and number of grains. Similar findings were documented by the different plant studies71,73. The growth parameter values were amplified by foliar application of (Ca + Mg) nanocomposite and abridged the cd toxicity in rice plants. These findings are reliable with previous studies that the application of CaO NPs improved the growth of barely plants and reduced the As toxicity74. Manzoor et al.75 described that iron oxide NPs upgraded the Cd toxicity and augmented the growth parameters in wheat plants through diminishing Cd uptake. The findings of this study showed that the application of rice husk biochar increased the growth parameter values in rice plants under Cd stress. This is concurrent with the aforementioned studies that the application of straw biochar augmented the growth parameters (plant height, number of tillers) of rice plants76. Apart from this, the outcomes of this research work depicted that the collective application of (Ca + Mg) nanocomposite and rice husk biochar significantly enhanced the growth parameter values in Cd contaminated soil in rice plants. These outcomes are steady with preceding research that the mutual execution of rice husk biochar and foliar spray of ZnO NPs pointedly increased the growth parameters in rice plants under Cd toxicity71. Shukla et al.77 also demonstrated that ZnO NPs in collaboration with biochar upsurge the growth parameters in rice plants beneath As stress.
In the present study, it was anticipated that SPAD meter values decreased by Cd contamination. Whereas Ca + Mg nanocomposite improved the SPAD meter values, which is consistent with previous results that the application of exogenous CaO NPs increased the SPAD meter values in barely seedlings by reducing Cd toxicity78. Correspondingly, findings showed that rice husk biochar application boosted the SPAD meter values in rice plants. These results are concurrent with prior studies that biochar application increased the SPAD meter values in paddy fields rice76. Besides this, the combined application of foliar spray of Ca + Mg nanocomposite and rice husk biochar significantly improved the SPAD meter values. Preceding studies presented that the foliar utilization of ZnO NPs and rice straw biochar significantly enhanced the SPAD meter values in sunflower plants under heavy metals stress72.
Plant photosynthesis and chlorophyll content are imperative indicators for growth and yield under acute ecological factors79. The existing outcomes established that Ca + Mg nanocomposite upgraded the chlorophyll level and gas exchange characteristics (Pn, Tr, WUE, and stomatal conductance) in rice leaves under Cd stress. Published literature highlighted that the application of CaO NPs alleviated the Cd toxicity and enhanced the chlorophyll content and photosynthetic capacity of Brassica plants80. A prior study also revealed that the application of TiO2 NPs amplified the growth, spike length and grain production by enhancing the photosynthesis efficiency and chlorophyll content of wheat plants bearing Cd toxicity7. The application of rice husk biochar amplified the chlorophyll contents and gas exchange traits in rice plants. Alike results were originated in an aforementioned study that testified that biochar application augmented the gas exchange characteristics (photosynthesis rate, transpiration rate, water usage efficacy, and stomatal conductance) and chlorophyll content in Phragmite karka plants81. Moreover, the chlorophyll contents and gas exchange attributes significantly increased when the foliar spray of Ca + Mg nanocomposite and rice husk biochar were applied. Previous studies also agreed that the collective application of rice straw biochar and ZnO NPs foliar spray enhanced plant development by increasing gas exchange characteristics and chlorophyll content values in maize plants bearing Cd toxicity82. Shukla et al.77 revealed that ZnO NPs foliar spray collectively with biochar increased the total chlorophyll, carotenoids and protein content in rice plants under flooded conditions. The above literature verified our results.
The production of antioxidants decreases the harmful effects of free radicals in plants. Numerous cases illustrated that Cd contamination increased the EL, MDA, and H2O2 values and diminished the antioxidant enzyme activities in the plants. Cadmium stress in rice and pea seedlings provokes the generation of plasma membrane-obliged NADPH oxidase in peroxisomes and consequences in the generation of ROS7,9. Similar findings were reported in a current study that the Cd contamination improved the EL, MDA, and H2O2 values and reduced the antioxidant enzyme activities (POD, SOD, APX, and CAT) in rice plants. Current research revealed that foliar utilization of Ca + Mg nanocomposite abridged the creation of reactive oxygen species (ROS) and improved the antioxidant enzyme activities (SOD, POD, APX, and CAT) in rice roots and shoots. Similar outcomes were found in a previous study that foliar spray of CaO NPs improved the antioxidant enzyme activities by reducing the generation of ROS species in Brassica napus plants83. Similarly, rice husk biochar application also increased the antioxidant characteristics and decreased the values of EL, MDA, and H2O2 in rice plants by alleviating Cd toxicity. It was evidenced from a prior study that rice husk biochar improved the maize plant growth by increasing antioxidant enzyme activities and decreasing the values of EL, and MDA. Rice husk biochar in paddy fields showed a promising mitigation effect on heavy metals contamination and plant development84,85. The foliar spray of Ca + Mg nanocomposite and rice husk biochar decreased the values of EL, MDA, and H2O2 in rice plants under Cd stress. These findings are concurrent with a prior study that demonstrated that the combined application of ZnO NPs foliar spray and biochar reduced the leaf electrolyte leakage (EL) and malondialdehyde (MDA) by reducing the uptake of As77. Rizwan et al.82 also evidenced that the collective usage of biochar and ZnO NPs foliar employment alleviates the Cd toxicity by improving antioxidant enzyme activities and abridged the making of ROS species in maize plants.
Cd toxicity increased the phenolic metabolites and total proteins in cucumber plants86. A preceding study showed that Cr toxicity enhanced total soluble proteins, total free amino acids, total soluble sugars, reducing sugars and phenolics in wheat plants87. The current study explained that Cd toxicity significantly increased the metabolite contents (TSP, TFAA, TSS and phenolics) in the roots and leaves of rice plants. The foliar spray of Ca-Mg nanocomposite slightly affects the TSP, TFAA, TSS, and phenolics in the roots and shoots of rice seedlings. Similarly, the application of rice husk biochar slightly affects the metabolite contents in the roots and shoots of rice plants. Likewise, the combined application of Ca + Mg nanocomposite and rice husk biochar moderately affects the TSP, TFAA, TSS and phenolics in the roots and shoots of rice plants.
Extreme Cd toxicity decreases the essential nutrients (Ca, Mg and K) in the roots and leaves of strawberry plants and restricts growth and development88. Collective application of SDZ and Cd significantly reduced the macro and micronutrients in the roots and shoots of spinach89. Foliar spray of collectively ZnO and MnO2 NPs improved the nutritive value of wheat grains by cumulative micronutrients (Zn, Fe, Ca, S, B), protein content and minimizing phytic acid content90. These findings were similar to our current study that Cd stress diminished the macro and micronutrients (Zn, Fe, Mg, Mn, K and Ca) in shoots and grains of rice seedlings. While foliar spray of Ca + Mg nanocomposite and biochar application enunciate an upsurge in the nutrient profile of rice shoots and grains.
Cd2+ as the most exchangeable chemical species among heavy metals that are adsorbed on the root epidermis and through xylem transport to shoots and grains, consequently increasing the Cd concentration in the root, shoot and grain of plants91. In this experiment, the foliar application of Ca + Mg nanocomposite decreased the Cd concentration in roots, shoots, as well as grains in the rice plants. Similar to previous research foliar application of Zn notably attenuated the Cd concentration in shoots and grains of rice plants through inhibiting Cd mobility in the xylem92. This study showed that applying rice husk biochar to passivate the Cd content in roots, shoots, as well as grains in paddy plants. It was similar to previous work that reported that rice husk biochar application in Cd contaminated soil increased the seedlings height, diameter, and biomass and alleviated the Cd concentration in oak plant tissues85,93. Chen et al.94 and Alaboudi et al.95 also observed that the application of rice husk biochar significantly decreased the translocation of Cd, Pb, Cu and Zn in maize plant tissues. Moreover, the present study also depicted that the mutual application of Ca + Mg nanocomposite foliar spray and rice husk biochar significantly suppressed the Cd contamination in roots, shoots, and grains in rice seedlings. It was consistent with previous research that the combined application of ZnO NPs foliar usage and rice husk biochar dramatically alleviated the Cd toxicity from roots, shoots, and grains of rice plants71. Seleiman et al.72 evidenced that synergistic use of rice husk biochar and ZnO NPs significantly reduced the heavy metals (Cd, Cr, Pb, and Cu) accumulation in sunflower plants.
Conclusion
The existing study inspected that the cadmium toxicity abridged plant growth, biomass and gas exchange characteristics in rice. Furthermore, Cd-stressed rice demonstrated increased MDA, H2O2, EL and O2•– contents, and enhanced the Cd concentration. However, alone Ca + Mg nanocomposite and rice husk biochar modified the activities of antioxidant system of treated rice via enhancement in the activity of CAT, SOD POD, and APX. Likewise, the collaborative usage of Ca + Mg nanocomposite and rice husk biochar supplementary enhanced the development and physiochemical attributes of rice cultivated in standard and Cd stress environments. Additional research work is mandatory to comprehend the molecular mechanisms contributing to Cd resistance in diverse crops by collaborative employment of Ca + Mg nanocomposite and rice husk biochar.
Acknowledgements
The authors wish to acknowledge the Institute of Coastal Environmental Pollution Control, Ministry of Education Key Laboratory of Marine Environment and Ecology, and College of Environmental Science and Engineering, Ocean University of China, Key Research and Development Program of Shandong Province (2022SFGC0302); Hainan Provincial Natural Science Foundation of China (423CXTD384) and Government College University, Faisalabad-Pakistan, to provide research facilities and financial support under project (5634/Punjab/NRPU/R&D/HEC/2016). The authors express their sincere appreciation to the researchers supporting project number (RSP2024R48), King Saud University, Riyadh, Saudi Arabia.
Author contributions
Fengmin Li and Shafaqat Ali: Supervision, Project administration, Resources, Funding acquisition, Writing - review & editing. Muhammad Azhar Ali: Conceptualization, Resources, Formal analysis, Data curation, Methodology, Writing - original draft, Writing - review & editing. Muhammad Nafees: Conceptualization, Resources, Writing - review & editing the final draft. Sarah Owdah Alomrani: Conceptualization, Software, Writing - review & editing. Yuanyuan Li: Formal analysis, Writing - review & editing. Qian Wang: Formal analysis, Data curation, Writing - review & editing. Mohammed Ali Alshehri and Khalid A. Al-Ghanim: Software, Writing - review & editing.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Ethical statement
This study does not include any research regarding animals and humans. Procedures used in this experiment was conducted by following guidelines provided by Department of Botany, GC, University Faisalabad-Punjab.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shafaqat Ali, Email: shafaqataligill@yahoo.com.
Fengmin Li, Email: lifengmin@ouc.edu.cn.
References
- 1.Li, Z. et al. Speciation, transportation, and pathways of cadmium in soil-rice systems: a review on the environmental implications and remediation approaches for food safety. Environ. Int. 156, 106749 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Zhang, L. et al. Co-application of biochar and nitrogen fertilizer promotes rice performance, decreases cadmium availability, and shapes rhizosphere bacterial community in paddy soil. Environ. Pollut. 308, 119624. 10.1016/j.envpol.2022.119624 (2022). [DOI] [PubMed] [Google Scholar]
- 3.McLaughlin, M., Smolders, E., Zhao, F., Grant, C. & Montalvo, D. Managing cadmium in agricultural systems. Adv. Agron. 166, 1–129 (2021). [Google Scholar]
- 4.Ullah, A. et al. Strategies for reducing cd concentration in paddy soil for rice safety. J. Clean. Prod. 316, 128116 (2021). [Google Scholar]
- 5.Li, J. et al. Immobilization of cadmium by mercapto-functionalized palygorskite under stimulated acid rain: Stability performance and micro-ecological response. Environ. Pollut. 306, 119400 (2022). [DOI] [PubMed] [Google Scholar]
- 6.Yang, J., Wang, J., Liao, X., Tao, H. & Li, Y. Chain modeling for the biogeochemical nexus of cadmium in soil–rice–human health system. Environ. Int. 167, 107424 (2022). [DOI] [PubMed] [Google Scholar]
- 7.Irshad, M. A. et al. Effect of green and chemically synthesized titanium dioxide nanoparticles on cadmium accumulation in wheat grains and potential dietary health risk: a field investigation. J. Hazard. Mater. 415, 125585. 10.1016/j.jhazmat.2021.125585 (2021). [DOI] [PubMed] [Google Scholar]
- 8.Shao, G., Huang, Q., An, H., Chen, J. & Li, X. Phosphorus application modulates cadmium toxicity in rice (Oryza sativa L.) by affecting the antioxidant defense system and regulating the expression of iron uptake/transport-related genes. Plant. Growth Regul. 102, 199–212 (2024). [Google Scholar]
- 9.Haider, F. U. et al. Cadmium toxicity in plants: impacts and remediation strategies. Ecotoxicol. Environ. Saf. 211, 111887. 10.1016/j.ecoenv.2020.111887 (2021). [DOI] [PubMed] [Google Scholar]
- 10.Ali, S. et al. Zinc fortification and alleviation of cadmium stress by application of lysine chelated zinc on different varieties of wheat and rice in cadmium stressed soil. Chemosphere. 295, 133829 (2022). [DOI] [PubMed] [Google Scholar]
- 11.Ghouri, F. et al. Polyploidy and zinc oxide nanoparticles alleviated cd toxicity in rice by modulating oxidative stress and expression levels of sucrose and metal-transporter genes. J. Hazard. Mater. 448, 130991. 10.1016/j.jhazmat.2023.130991 (2023). [DOI] [PubMed] [Google Scholar]
- 12.Yang, Y. et al. Historical and future trends of cadmium in rice soils deduced from long-term regional investigation and probabilistic modeling. J. Hazard. Mater. 415, 125746 (2021). [DOI] [PubMed] [Google Scholar]
- 13.Adil, M. F. et al. Stress signaling convergence and nutrient crosstalk determine zinc-mediated amelioration against cadmium toxicity in rice. Ecotoxicol. Environ. Saf. 230, 113128 (2022). [DOI] [PubMed] [Google Scholar]
- 14.Mapodzeke, J. M. et al. Myriad of physio-genetic factors determining the fate of plant under zinc nutrient management. Environ. Exp. Bot. 189, 104559. 10.1016/j.envexpbot.2021.104559 (2021). [Google Scholar]
- 15.Zeshan, A. et al. Improvement of morpho-physiological, ultrastructural and nutritional profiles in wheat seedlings through astaxanthin nanoparticles alleviating the cadmium toxicity. J. Hazard. Mater. 424, 126511 (2022). [DOI] [PubMed] [Google Scholar]
- 16.Hussain, B. et al. Foliage application of selenium and silicon nanoparticles alleviates Cd and Pb toxicity in rice (Oryza sativa L). Sci. Total Environ. 712, 136497 (2020). [DOI] [PubMed] [Google Scholar]
- 17.Mlangeni, A. T. et al. Safety of African grown rice: comparative review of as, cd, and pb contamination in African rice and paddy fields. Heliyon. 9, e18314. 10.1016/j.heliyon.2023.e18314 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lou, F. et al. Different composites inhibit cd accumulation in grains under the rice-oilseed rape rotation mode of karst area: a field study. Ecotoxicol. Environ. Saf. 256, 114884. 10.1016/j.ecoenv.2023.114884 (2023). [DOI] [PubMed] [Google Scholar]
- 19.Badihi, L., Gerami, M., Akbarinodeh, D., Shokrzadeh, M. & Ramezani, M. Physio-chemical responses of exogenous calcium nanoparticle and putrescine polyamine in Saffron (Crocus sativus L). Physiol. Mol. Biol. Plants. 27, 119–133 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aziz, Y., Shah, G. A. & Rashid, M. I. ZnO nanoparticles and zeolite influence soil nutrient availability but do not affect herbage nitrogen uptake from biogas slurry. Chemosphere. 216, 564–575 (2019). [DOI] [PubMed] [Google Scholar]
- 21.Upadhyaya, H., Begum, L., Dey, B., Nath, P. & Panda, S. Impact of calcium phosphate nanoparticles on rice plant. J. Plant. Sci. Phytopathol. 1 1), 001–010 (2017). [Google Scholar]
- 22.Shaul, O. Magnesium transport and function in plants: the tip of the iceberg. Biometals. 15, 307–321 (2002). [DOI] [PubMed] [Google Scholar]
- 23.Tang, R. J. & Luan, S. Regulation of calcium and magnesium homeostasis in plants: from transporters to signaling network. Curr. Opin. Plant. Biol. 39, 97–105 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Nguyen, T. T. N. et al. The effects of short term, long term and reapplication of biochar on soil bacteria. Sci. Total Environ. 636, 142–151 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Hussain, M. et al. Biochar for crop production: potential benefits and risks. J. Soil. Sediment. 17, 685–716 (2017). [Google Scholar]
- 26.Lu, K. et al. Effect of bamboo and rice straw biochars on the mobility and redistribution of heavy metals (cd, Cu, Pb and Zn) in contaminated soil. J. Environ. Manag. 186, 285–292 (2017). [DOI] [PubMed] [Google Scholar]
- 27.Oladele, S., Adeyemo, A. & Awodun, M. Influence of rice husk biochar and inorganic fertilizer on soil nutrients availability and rain-fed rice yield in two contrasting soils. Geoderma. 336, 1–11 (2019). [Google Scholar]
- 28.Lustosa Filho, J. F. et al. Aging of biochar-based fertilizers in soil: effects on phosphorus pools and availability to Urochloa brizantha grass. Sci. Total Environ. 709, 136028 (2020). [DOI] [PubMed] [Google Scholar]
- 29.Nafees, M. et al. Effect of nanoparticles on Plant Growth and Physiology and on soil microbes. In: (eds Bhushan, I., Singh, V. & Tripathi, D.) Nanomaterials and Environmental Biotechnology. Nanotechnology in the Life Sciences. Springer, Cham. 10.1007/978-3-030-34544-0_5. (2020). [Google Scholar]
- 30.Nafees, M., Ali, S., Naveed, M. & Rizwan, M. Efficiency of biogas slurry and Burkholderia phytofirmans PsJN to improve growth, physiology, and antioxidant activity of Brassica napus L. in chromium-contaminated soil. Environ. Sci. Pollut Res. 25, 6387–6397. 10.1007/s11356-017-0924-z (2018). [DOI] [PubMed] [Google Scholar]
- 31.Bouyoucos, G. J. Hydrometer method improved for making particle size analyses of soils 1. Agron. J. 54 5), 464–465 (1962). [Google Scholar]
- 32.Amacher, M. C. Nickel, cadmium, and lead. Methods Soil. Analysis: Part. 3 Chem. Methods. 5, 739–768 (1996). [Google Scholar]
- 33.Moodie, C., Smith, H. & McCreery, R. Laboratory manual for soil fertility. Soil. Sci. 71, 5, 400 (1951). [Google Scholar]
- 34.Jackson, M. Interlayering of expansible layer silicates in soils by chemical weathering. Clays Clay Min. 11, 29–46 (1962). [Google Scholar]
- 35.Irfan, M. et al. Effect of wheat straw derived biochar on the bioavailability of pb, cd and cr using maize as test crop. J. Saudi Chem. Soc. 25 5), 101232 (2021). [Google Scholar]
- 36.Ali, M. A. et al. Modulation of cd carriers by innovative nanocomposite (ca + mg) and Cd-resistance microbes (Bacillus pumilus): a mechanistic approach to enhance growth and yield of rice (Oryza sativa L). Front. Plant. Sci. 15, 1387187. 10.3389/fpls.2024.1387187 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lichtenthaler, H. K. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol. 148, 350–382 (1987). [Google Scholar]
- 38.Zhang, J. & Kirkham, M. Drought-stress-induced changes in activities of superoxide dismutase, catalase, and peroxidase in wheat species. Plant. Cell. Physiol. 35 5), 785–791 (1994). [Google Scholar]
- 39.Abbas, A., Ahmad, M. S. A., Ashraf, M., Ali, Q. & Alvi, A. K. Role of antioxidative defense system in amelioration of cadmium-induced phytotoxic effects in germinating seeds of maize (Zea mays). Crop Pasture Sci. 73 5), 599–613 (2021). [Google Scholar]
- 40.Dionisio-Sese, M. L. & Tobita, S. Antioxidant responses of rice seedlings to salinity stress. Plant. Sci. 135 1), 1–9 (1998). [Google Scholar]
- 41.Jana, S. & Choudhuri, M. A. Senescence in submerged aquatic angiosperms: effects of heavy metals. New. Phytol. 90 3), 477–484 (1982). [Google Scholar]
- 42.Yang, H. Y., Shi, G. X., Qiao, X. Q. & Tian, X. L. Exogenous spermidine and spermine enhance cadmium tolerance of Potamogeton malaianus. Russ J. Plant. Physiol. 58, 622–628 (2011). [Google Scholar]
- 43.Zhang, X. Z. The measurement and mechanism of lipid peroxidation and SOD, POD and CAT activities in biological system. Res. Methodol. crop Physiol. 208–211 (1992).
- 44.Aebi, H. Catalase in vitro. Methods in enzymology, Vol. 105, 121–126 10.1016/s0076-6879(84)05016-3 (Elsevier, 1984). [DOI] [PubMed]
- 45.Nakano, Y. & Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant. Cell. Physiol. 22 5), 867–880 (1981). [Google Scholar]
- 46.Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72 1–2), 248–254 (1976). [DOI] [PubMed] [Google Scholar]
- 47.Hamilton, P. B., Van Slyke, D. D. & Lemish, S. The gasometric determination of free amino acids in blood filtrates by the ninhydrin-carbon dioxide method. J. Biol. Chem. 150, 231–250 (1943). [Google Scholar]
- 48.Wolfe, K., Wu, X. & Liu, R. H. Antioxidant activity of apple peels. J. Agric. Food Chem. 51 3), 609–614 (2003). [DOI] [PubMed] [Google Scholar]
- 49.Yemm, E. W. & Willis, A. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 57 3), 508 (1954). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lwalaba, J. L. W. et al. Physiological and molecular mechanisms of cobalt and copper interaction in causing phyto-toxicity to two barley genotypes differing in Co tolerance. Ecotoxicol. Environ. Saf. 187, 109866 (2020). [DOI] [PubMed] [Google Scholar]
- 51.Ahmad, P. et al. Alleviation of cadmium toxicity in Brassica juncea L. (Czern. & Coss.) by calcium application involves various physiological and biochemical strategies. PLoS ONE 10(1), e0114571 (2015). [DOI] [PMC free article] [PubMed]
- 52.Xiong, C. et al. Investigation on the efficiency and mechanism of cd (II) and pb (II) removal from aqueous solutions using MgO nanoparticles. J. Hazard. Mater. 299, 664–674 (2015). [DOI] [PubMed] [Google Scholar]
- 53.Wang, M. Y. et al. Cadmium oral bioavailability is affected by calcium and phytate contents in food: evidence from leafy vegetables in mice. J. Hazard. Mater. 424, 127373 (2022). [DOI] [PubMed] [Google Scholar]
- 54.Ur Rehman, M. Z. et al. Effect of acidified biochar on bioaccumulation of cadmium (cd) and rice growth in contaminated soil. Environ. Technol. Innov. 19, 101015 (2020). [Google Scholar]
- 55.Song, W. Y. et al. Relationship between calcium decoding elements and plant abiotic-stress resistance. Int. J. Biol. Sci. 4 2), 116 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sarwat, M., Ahmad, P., Nabi, G. & Hu, X. Ca2+ signals: the versatile decoders of environmental cues. Crit. Rev. Biotechnol. 33 1), 97–109 (2013). [DOI] [PubMed] [Google Scholar]
- 57.Tuteja, N. & Sopory, S. K. Chemical signaling under abiotic stress environment in plants. Plant Signal. Behav. 3 8), 525–536 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang, T. & Poovaiah, B. W. Calcium/calmodulin-mediated signal network in plants. Trends Plant. Sci. 8 10), 505–512 (2003). [DOI] [PubMed] [Google Scholar]
- 59.DalCorso, G., Farinati, S. & Furini, A. Regulatory networks of cadmium stress in plants. Plant. Signal. Behav. 5 6), 663–667 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cakmak, I. & Kirkby, E. A. Role of magnesium in carbon partitioning and alleviating photooxidative damage. Physiol. Plant. 133 4), 692–704 (2008). [DOI] [PubMed] [Google Scholar]
- 61.Mengutay, M., Ceylan, Y., Kutman, U. B. & Cakmak, I. Adequate magnesium nutrition mitigates adverse effects of heat stress on maize and wheat. Plant. Soil. 368, 57–72 (2013). [Google Scholar]
- 62.Gerendás, J. & Führs, H. The significance of magnesium for crop quality. Plant. Soil. 368, 101–128 (2013). [Google Scholar]
- 63.O’Connor, D. et al. Biochar application for the remediation of heavy metal polluted land: a review of in situ field trials. Sci. Total Environ. 619, 815–826 (2018). [DOI] [PubMed] [Google Scholar]
- 64.Liu, X. et al. Biochar’s effect on crop productivity and the dependence on experimental conditions—a meta-analysis of literature data. Plant. Soil. 373, 583–594 (2013). [Google Scholar]
- 65.Ali, S. et al. Biochar soil amendment on alleviation of drought and salt stress in plants: a critical review. Environ. Sci. Pollut Res. 24, 12700–12712 (2017). [DOI] [PubMed] [Google Scholar]
- 66.Kolton, M., Graber, E. R., Tsehansky, L., Elad, Y. & Cytryn, E. Biochar-stimulated plant performance is strongly linked to microbial diversity and metabolic potential in the rhizosphere. New. Phytol.. 213 3), 1393–1404 (2017). [DOI] [PubMed] [Google Scholar]
- 67.Li, R. et al. Recovery of phosphate and dissolved organic matter from aqueous solution using a novel CaO-MgO hybrid carbon composite and its feasibility in phosphorus recycling. Sci. Total Environ. 642, 526–536 (2018). [DOI] [PubMed] [Google Scholar]
- 68.Wu, C. et al. Effect of sulfur-iron modified biochar on the available cadmium and bacterial community structure in contaminated soils. Sci. Total Environ. 647, 1158–1168 (2019). [DOI] [PubMed] [Google Scholar]
- 69.Li, R. et al. High-efficiency removal of pb (II) and humate by a CeO2–MoS2 hybrid magnetic biochar. Bioresour Technol. 273, 335–340 (2019). [DOI] [PubMed] [Google Scholar]
- 70.Danso, O. P. et al. The management of cd in rice with biochar and selenium: effects, efficiency, and practices. Carbon Res. 2 1), 41 (2023). [Google Scholar]
- 71.Ali, S. et al. Combined use of biochar and zinc oxide nanoparticle foliar spray improved the plant growth and decreased the cadmium accumulation in rice (Oryza sativa L.) plant. Environ. Sci. Pollut Res. 26, 11288–11299 (2019). [DOI] [PubMed] [Google Scholar]
- 72.Seleiman, M. F. et al. Effects of ZnO nanoparticles and biochar of rice straw and cow manure on characteristics of contaminated soil and sunflower productivity, oil quality, and heavy metals uptake. Agron. 10 6), 790 (2020). [Google Scholar]
- 73.Azhar, M. et al. Comparative effectiveness of different biochars and conventional organic materials on growth, photosynthesis and cadmium accumulation in cereals. Chemosphere. 227, 72–81 (2019). [DOI] [PubMed] [Google Scholar]
- 74.Nazir, M. M. et al. Calcium oxide nanoparticles have the role of alleviating arsenic toxicity of barley. Front. Plant. Sci. 13, 843795 (2022a). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Manzoor, N. et al. Iron oxide nanoparticles ameliorated the cadmium and salinity stresses in wheat plants, facilitating photosynthetic pigments and restricting cadmium uptake. Sci. Total Environ. 769, 145221 (2021). [DOI] [PubMed] [Google Scholar]
- 76.Li, H. et al. Response of soil nutrients retention and rice growth to biochar in straw returning paddy fields. Chemosphere. 312, 137244. 10.1016/j.chemosphere.2022.137244 (2023). [DOI] [PubMed] [Google Scholar]
- 77.Shukla, K., Khanam, R., Biswas, J. K. & Srivastava, S. Zinc oxide nanoparticles in combination with biochar alleviate arsenic accumulation in field grown rice (Oryza sativa L.) crop. Rhizosphere. 27, 100764. 10.1016/j.rhisph.2023.100764 (2023). [Google Scholar]
- 78.Nazir, M. M. et al. Exogenous calcium oxide nanoparticles alleviate cadmium toxicity by reducing cd uptake and enhancing antioxidative capacity in barley seedlings. J. Hazard. Mater. 438, 129498 (2022b). [DOI] [PubMed] [Google Scholar]
- 79.Hussain, S. et al. Photosynthesis research under climate change. Photosynth. Res. 150, 5–19 (2021). [DOI] [PubMed] [Google Scholar]
- 80.Mubeen, S. et al. Calcium nanoparticles impregnated with benzenedicarboxylic acid: a new approach to alleviate combined stress of DDT and cadmium in Brassica alboglabra by modulating bioacummulation, antioxidative machinery and osmoregulators. Front. Plant. Sci. 13, 825829 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Abideen, Z., Koyro, H. W., Huchzermeyer, B., Gul, B. & Khan, M. A. Impact of a biochar or a biochar-compost mixture on water relation, nutrient uptake and photosynthesis of Phragmites karka. Pedosphere. 30(4), 466–477. 10.1016/S1002-0160(17)60362-X (2020). [Google Scholar]
- 82.Rizwan, M. et al. Alleviation of cadmium accumulation in maize (Zea mays L.) by foliar spray of zinc oxide nanoparticles and biochar to contaminated soil. Environ. Pollut. 248, 358–367 (2019). [DOI] [PubMed] [Google Scholar]
- 83.Ayyaz, A. et al. Calcium nanoparticles (Ca-NPs) improve drought stress tolerance in Brassica napus by modulating the photosystem II, nutrient acquisition and antioxidant performance. NanoImpact. 28, 100423 (2022). [DOI] [PubMed] [Google Scholar]
- 84.Kim, H. S. et al. Effect of biochar on reclaimed tidal land soil properties and maize (Zea mays L.) response. Chemosphere. 142, 153–159. 10.1016/j.chemosphere.2015.06.041 (2016). [DOI] [PubMed] [Google Scholar]
- 85.Asadi, H. et al. Application of rice husk biochar for achieving sustainable agriculture and environment. Rice Sci. 28(4), 325–343. 10.1016/j.rsci.2021.05.004 (2021). [Google Scholar]
- 86.Dresler, S. et al. Allantoin attenuates cadmium-induced toxicity in cucumber plants. Ecotoxicol. Environ. Saf. 170, 120–126 (2019). [DOI] [PubMed] [Google Scholar]
- 87.Alam, R., Rasheed, R., Ashraf, M. A., Hussain, I. & Ali, S. Allantoin alleviates chromium phytotoxic effects on wheat by regulating osmolyte accumulation, secondary metabolism, ROS homeostasis and nutrient acquisition. J. Hazard. Mater. 131920 2023). [DOI] [PubMed]
- 88.Muradoglu, F. et al. Cadmium toxicity affects chlorophyll a and b content, antioxidant enzyme activities and mineral nutrient accumulation in strawberry. Biol. Res. 48 1), 1–7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nafees, M. et al. Mechanism and synergistic effect of sulfadiazine (SDZ) and cadmium toxicity in spinach (Spinacia oleracea L.) and its alleviation through zinc fortification. J. Hazard. Mater. 464, 132903. 10.1016/j.jhazmat.2023.132903 (2024). [DOI] [PubMed] [Google Scholar]
- 90.Lian, J. et al. Foliar spray of combined metal-oxide nanoparticles alters the accumulation, translocation and health risk of cd in wheat (Triticum aestivum L). J. Hazard. Mater. 440, 129857 (2022). [Google Scholar]
- 91.Li, Y. et al. Toxic effects of cadmium on the physiological and biochemical attributes of plants, and phytoremediation strategies: a review. Environ. Pollut. 325, 121433. 10.1016/j.envpol.2023.121433 (2023). [DOI] [PubMed] [Google Scholar]
- 92.Zheng, S. et al. Foliar zinc reduced cd accumulation in grains by inhibiting cd mobility in the xylem and increasing cd retention ability in roots1. Environ. Pollut. 333, 122046. 10.1016/j.envpol.2023.122046 (2023). [DOI] [PubMed] [Google Scholar]
- 93.Amirahmadi, E., Mohammad Hojjati, S., Kammann, C., Ghorbani, M. & Biparva, P. The potential effectiveness of biochar application to reduce soil cd bioavailability and encourage oak seedling growth. Appl. Sci. 10 10), 3410 (2020). [Google Scholar]
- 94.Chen, D. et al. Effects of biochar on availability and plant uptake of heavy metals–A meta-analysis. J. Environ. Physiol. Plant. Manag. 222, 76–85 (2018). [DOI] [PubMed] [Google Scholar]
- 95.Alaboudi, K. A., Ahmed, B. & Brodie, G. Effect of biochar on pb, cd and cr availability and maize growth in artificial contaminated soil. Ann. Agric. Sci. 64 1), 95–102 (2019). [Google Scholar]
- 96.Ahmed, T. et al. Green magnesium oxide nanoparticles-based modulation of cellular oxidative repair mechanisms to reduce arsenic uptake and translocation in rice (Oryza sativa L.) plants. Environ. Pollut. 288, 117785 (2021). [DOI] [PubMed] [Google Scholar]
- 97.Adrees, M. et al. Simultaneous mitigation of cadmium and drought stress in wheat by soil application of iron nanoparticles. Chemosphere. 238, 124681 (2020). [DOI] [PubMed] [Google Scholar]
- 98.Faiz, S. et al. Role of magnesium oxide nanoparticles in the mitigation of lead-induced stress in Daucus carota: modulation in polyamines and antioxidant enzymes. Int. J. Phytoremediation. 24 4), 364–372 (2022). [DOI] [PubMed] [Google Scholar]
- 99.Wei, X. et al. A field study of nano-FeS loaded lignin hydrogel application for cd reduction, nutrient enhancement, and microbiological shift in a polluted paddy soil. Chem. Eng. J. 451, 138647 (2023). [Google Scholar]
- 100.Zhou, P. et al. Lynch, I. Iron-based nanomaterials reduce cadmium toxicity in rice (Oryza sativa L.) by modulating phytohormones, phytochelatin, cadmium transport genes and iron plaque formation. Environ. Pollut. 320, 121063 (2023). [DOI] [PubMed] [Google Scholar]
- 101.Manzoor, N. et al. Silicon oxide nanoparticles alleviate chromium toxicity in wheat (Triticum aestivum L). Environ. Pollut. 315, 120391 (2022). [DOI] [PubMed] [Google Scholar]
- 102.Sun, Y. et al. New insights in to the ameliorative effects of zinc and iron oxide nanoparticles to arsenic stressed spinach (Spinacia oleracea L). Plant Physiol. Biochem. 199, 107715 (2023). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.