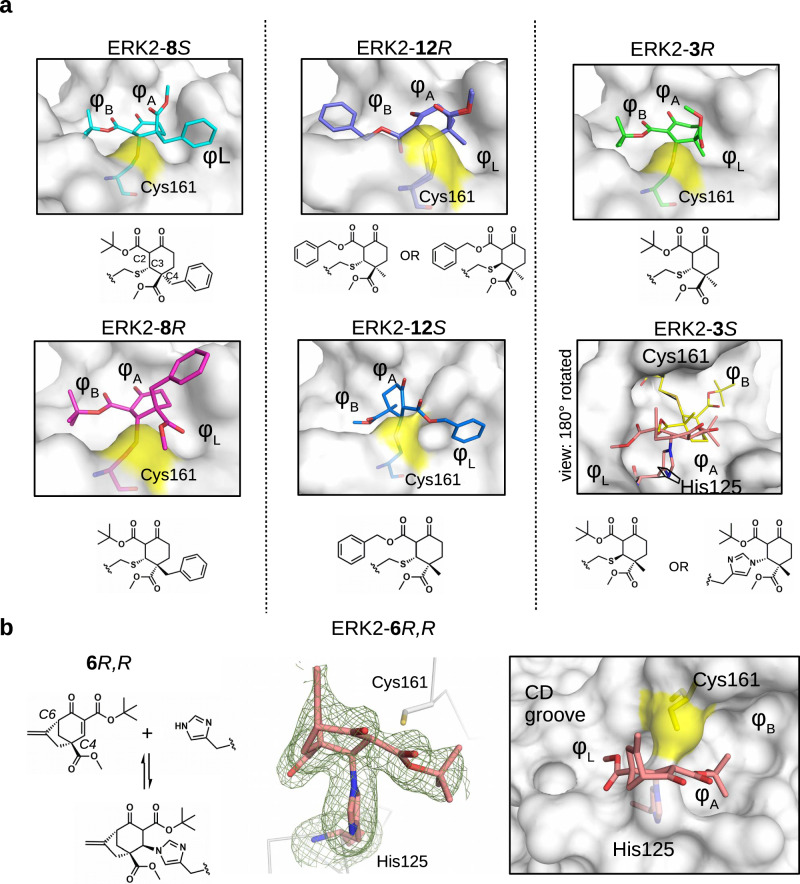
Fig. 3. Structural comparison of the ERK2 covalent adducts with different cyclohexenone containing compounds.
a Panels show the crystallographic complexes of ERK2-8S or -8R, ERK2-12R or -12S, and ERK2-3R or -3S (PDB ID: 8PSW, 8PSY, 8PT0, 8PT1, 8PST, 8PT5, respectively). The absolute configuration at C4 is R or S; 8S/8 R, 12 R/12S and 3 R/3S are enantiomeric pairs but new asymmetric centers form upon adduct formation at C3 and C2, too. The cysteine adduct in the ERK2-12R complex has two alternative C-S covalent bond conformations (see Supplementary Fig. 14). The ERK2 D-groove is shown with surface representation from the same view for 8S/8 R, 12 R/12S and 3 R (and the atoms of Cys161 on the surface are colored yellow). Note that, the crystal structure of the ERK2-3S complex is shown in a view 180° rotated along the Z axis (perpendicular to the plane of the panel) compared to the other surface panels. This latter complex has a cysteine (yellow) as well as a histidine adduct (salmon) (and the D-groove is shown in surface representation apart from the amino acid adducts, which are shown in sticks). Naturally, one molecule can form only one of the two adducts, and the final structural model fitted best with the crystallographic data if the two alternative adducts were equally present. (The conformation of the intact cysteine or the histidine side chain is shown in black.). b 6 R,R forms a reversible covalent adduct with the imidazole side chain of histidine in the MAPK D-groove (see Supplementary Fig. 14). The crystal structure of the ERK2-6R,R complex (PDB ID: 8PT3) with the Fo-Fc simulated annealing omit map shown at 2ϭ for the histidine adduct is shown in the middle panel. The panel on the right shows the MAPK docking groove in surface representation highlighting the different hydrophobic pockets (φA, φB, φL) and the polar CD groove.