Table 4.
Summary of apparent binding affinities for different C4-extended cyclohexenone compounds
| ERK2 Kiapp/µM | p38 Kiapp/µM | JNK1 Kiapp/µM | logP | ||
|---|---|---|---|---|---|
| 28S | 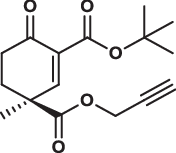 |
1.0 ± 0.1 | 0.8 ± 0.6 | 3.4 ± 0.5 | 1.800 |
| 28R | 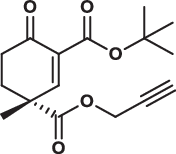 |
3.8 ± 0.2 | 3.5 ± 0.2 | 17.0 ± 2.0 | 1.800 |
| 29S | 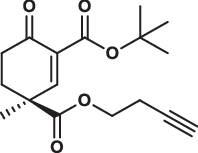 |
1.4 ± 0.2 | 1.1 ± 0.1 | 2.4 ± 0.3 | 2.190 |
| 30S | 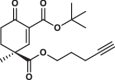 |
0.8 ± 0.1 | 0.9 ± 0.2 | 1.7 ± 0.4 | 2.580 |
| 31S | 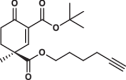 |
1.0 ± 0.1 | 1.3 ± 0.0 | 1.6 ± 0.4 | 2.970 |
| 32S | 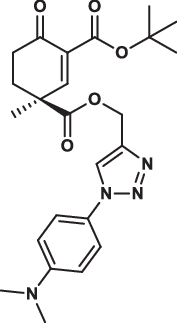 |
2.3 ± 0.1 | 3.5 ± 0.9 | 2.4 ± 0.8 | 3.014 |
| 32R | 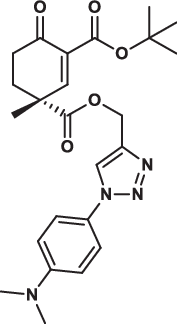 |
15.7 ± 2.9 | 12.8 ± 3.0 | 13.6 ± 6.1 | 3.014 |
| 33S | 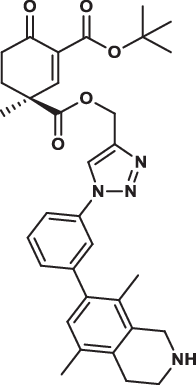 |
4.4 ± 0.7 | 2.8 ± 0.7 | 8.8 ± 2.6 | 4.877 |
| 34S | 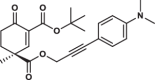 |
-* | 3.1 ± 0.5 | 1.3 ± 0.6 | 3.285 |
| 34R | 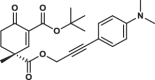 |
-* | 3.9 ± 0.8 | 2.4 ± 0.9 | 3.285 |
| 35S | 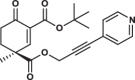 |
1.0 ± 0.0 | 1.5 ± 0.3 | 2.7 ± 0.6 | 2.614 |
| 35R | 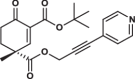 |
3.8 ± 0.2 | 1.8 ± 0.7 | 2.9 ± 1.1 | 2.614 |
Data show the mean ± parameter error estimates from weighted least square method, see Supplementary Fig. 19; logP are calculated values. (* indicates only partial inhibition, suggesting weak binding).
