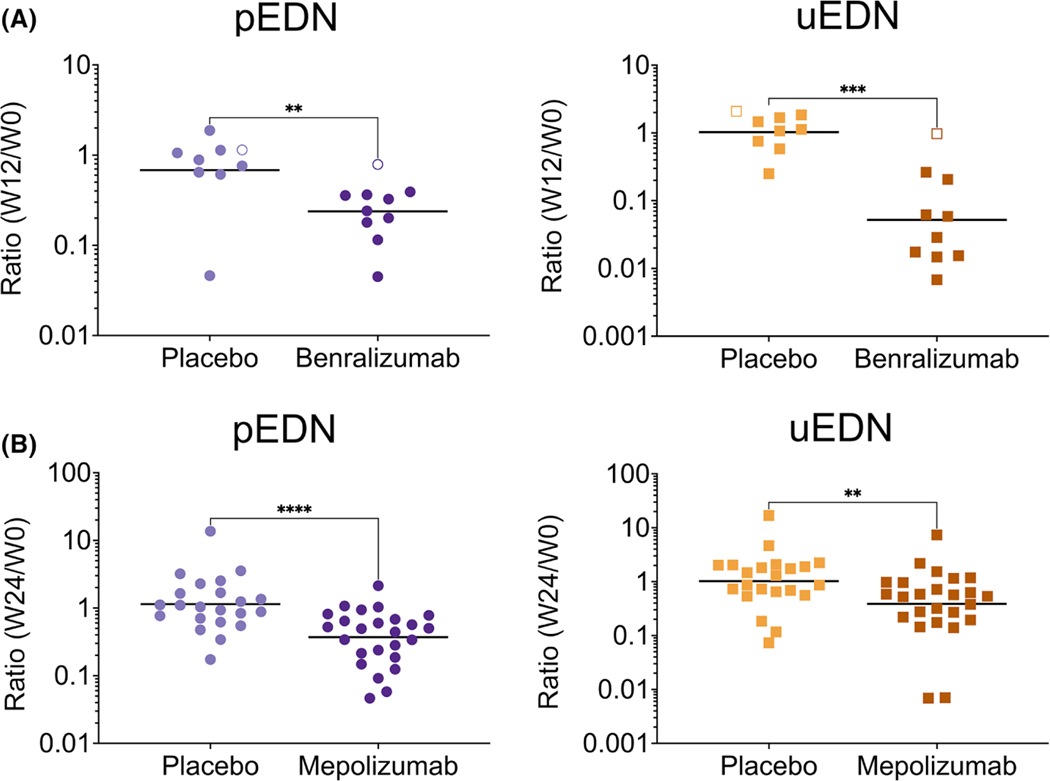
FIGURE 3.
The plasma ratio and uEDN ratio significantly decrease in response to benralizumab and mepolizumab compared to placebo. The EDN ratio was assessed from plasma (**p < 0.01, ****p < 0.0001) and urine (**p < 0.01, ***p < 0.001) collected from (A) PDGFRA-negative HES patients at baseline and week 12 receiving placebo (N = 9), or benralizumab (N = 10), the open symbols represent nonresponders, and (B) EGPA patients at baseline and week 24 receiving placebo (N = 22) or mepolizumab (N = 25)