Figure 3.
Procedure for in vivo electrophysiological recording combined with optogenetics
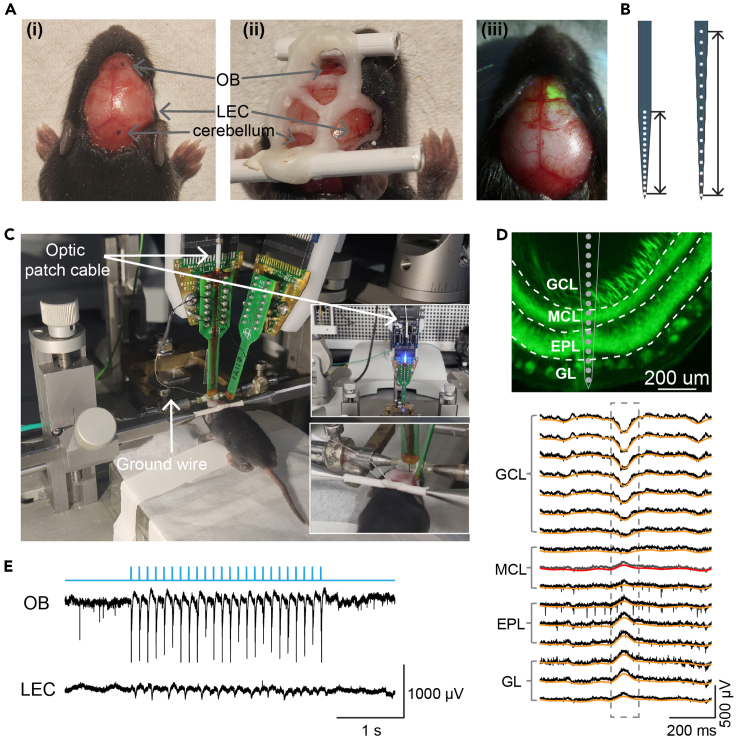
(A) Anesthetized neonatal mouse with recording locations marked (i) and exposed (ii) for multi-site extracellular recordings. The two plastic bars were mounted on the nasal and occipital bones respectively with the dental cement for tight head fixation during the recording. (iii) Overlaid photograph (from pictures taken under 440 nm blue light and neutral white light) showing the expression of hChR2-EYFP in the OB through the surface of the brain.
(B) Schematic reconstruction of extracellular recording electrode tips with 50 μm (left) and 100 μm (right) inter-site spacing. Scale bars correspond to 750 μm (left) and 1500 μm (right) respectively.
(C) Head-fixed neonatal mouse in the stereotaxic apparatus with optrode inserted in OB and electrode inserted in LEC. The optrode is attached to the laser delivery system (upper inner photo) for optogenetic stimulation. The silver wire is positioned into the cerebellum as ground and reference (lower inner photo).
(D) Digital post-mortem photomontage reconstructing the location of the 16 recording channels of optrode in ventral OB of a P11 hChR2-EYFP expressing mouse (top; GCL: granule cell layer; MCL: mitral cell layer; EPL: external plexiform layer; GL: glomerular layer), and the laminar spontaneous activity across the corresponding OB layers (down; raw recording traces in black, 1–12 Hz band-pass filtered LFP traces in orange). Note that the 1–12 Hz LFPs reverse at the channel below the first MCL recording channel (traces in gray and red).
(E) Representative traces of simultaneous extracellular recordings in OB and LEC with both LFPs and MUA during blue light (473 nm) pulse stimulation at 8 Hz. The TTL signals indicating 3-ms light square pulses are marked in light blue.