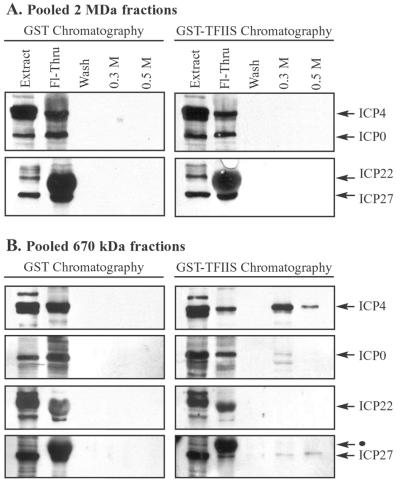
FIG. 10.
TFIIS affinity chromatography showing viral IE proteins in low-molecular-mass fractions containing RNAPII. Nuclear extracts, prepared from cells infected with wild-type HSV-1, were fractionated by Sepharose CL-2B gel filtration chromatography, as described for Fig. 2. (A) Fractions surrounding the 2-MDa peak (indicated in Fig. 2, 3, and 9) were pooled and subjected to GST and GST-TFIIS affinity chromatography. Bound proteins were eluted sequentially with buffers containing 0.3 and 0.5 M NaCl. Eluates were concentrated and analyzed by immunoblotting with antibodies that recognize the IE proteins ICP4, ICP0, ICP22, and ICP27. Each blot contained a lane of nuclear extract (20 μg) to indicate the position of each IE protein. The Fl-Thru lane contained one-quarter of the flowthrough, and the wash lane contained one-half of the wash. (B) Fractions at and below ∼670 kDa (indicated in Fig. 2, 3, and 9) were pooled and subjected to GST and GST-TFIIS affinity chromatography. Bound proteins were eluted and analyzed as described for panel A. The black dot indicates an ICP22 band above the ICP27 band, visible from a previous probing of the Western blot.