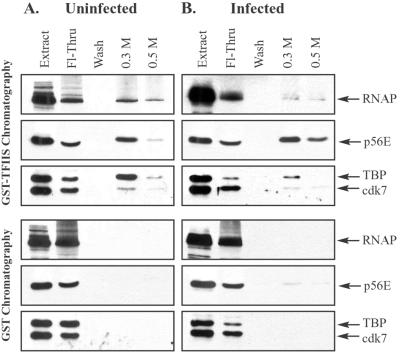
FIG. 7.
TFIIS affinity chromatography of pooled low-molecular-mass fractions from gel filtration chromatography. Nuclear extracts prepared from uninfected or infected cells were fractionated by Sepharose CL-2B gel filtration chromatography, as described for Fig. 2. Fractions at and below ∼670 kDa (indicated in Fig. 2 and 3) were pooled and chromatographed on GST and GST-TFIIS columns. Bound proteins were eluted sequentially with buffers containing 0.3 and 0.5 M NaCl. Eluates were concentrated and analyzed by immunoblotting with antibodies that recognize the large subunit of RNAP II, the p56E subunit of TFIIE, the TBP subunit of TFIID, and the cdk7 subunit of TFIIH. Each blot contained a lane of uninfected (A) or infected (B) nuclear extract (20 μg each) to indicate positions of GTFs, as well as a lane containing one-quarter of the flowthrough (Fl-Thru) and a lane containing one-half of the wash.