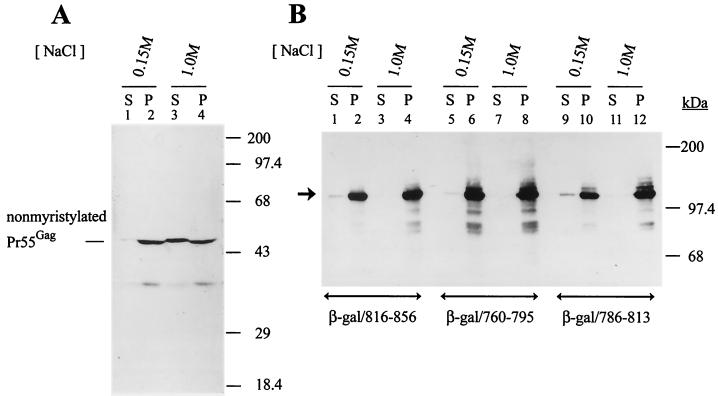
FIG. 5.
Resistance of membrane-bound β-galactosidase/LLP fusion proteins to high-salt extraction. (A) High-salt extraction of nonmyristylated Pr55Gag from membranes. The postnuclear supernatant prepared from COS-1 cells transfected with HIVgptmyr− was divided into two portions and then ultracentrifuged to obtain the membranes. The pelleted membranes were incubated with phosphate buffer, pH 7.4, supplemented with 0.15 or 1 M NaCl as indicated, and the resultant mixtures were ultracentrifuged to yield soluble (S) and membrane-bound (P) fractions. Equal volumes of the soluble and membrane fractions were analyzed by Western blotting using CA MAb. (B) Resistance of the β-galactosidase/LLP fusion proteins to high-salt extraction. Membrane pellets prepared from COS-1 cells expressing various β-galactosidase/LLP fusion proteins were extracted with low or high salt. The extracted mixtures were ultracentrifuged to resolve them into soluble and membrane fractions and then were analyzed by Western blotting using the β-galactosidase MAb. The arrowhead indicates the migration of the β-galactosidase/LLP fusion proteins.