Figure 2: Protection from patents and regulatory exclusivities on GLP-1 receptor agonists, 1993-2038.
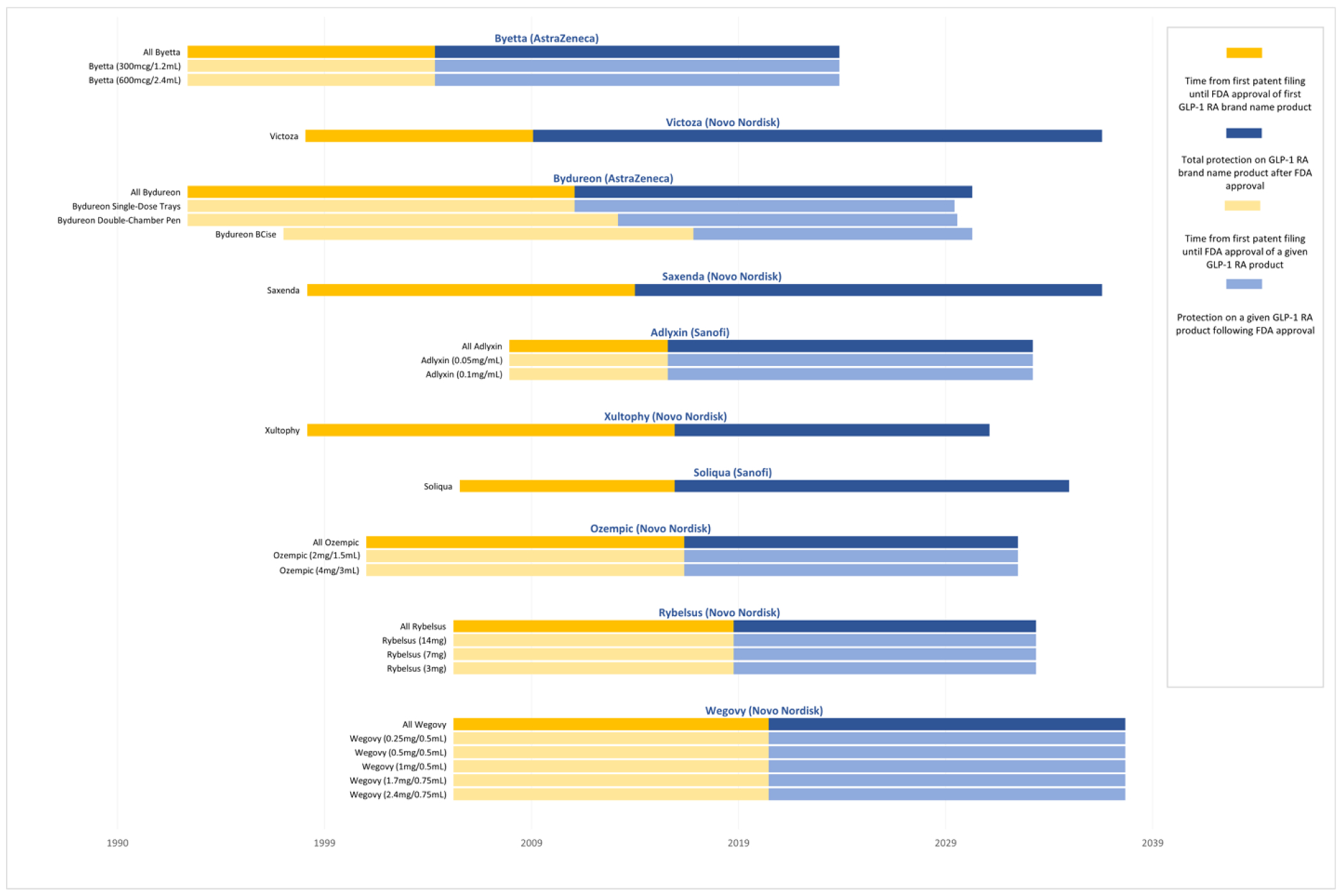
This figure shows the expected duration of protection from generic competition on each GLP-1 receptor agonist from the time of first patent filing until the expiration of the last patent or regulatory exclusivity. The dark blue bars (uppermost for each product) represent protection for the product as a whole, while the light blue bars represent protection for each of the product’s individual strengths and/or formulations. Products are listed in ascending order based on the initial Food and Drug Administration (FDA) approval date for a given product. Manufacturers may add new patents in subsequent years, which could expire later than patents depicted in the figure. The median total duration of protection from FDA approval among GLP-1 receptor agonists is 18.3 years (IQR: 16.0-19.4). The median time elapsed from the earliest patent filing date within a given product to the expiration date of the last-to-expire patent or exclusivity on that product is 31.9 years (IQR: 29.9-36.6).
