Figure 1. Reconstitution of tripartite Ssq1*-Hsc20-Isu1 complex.
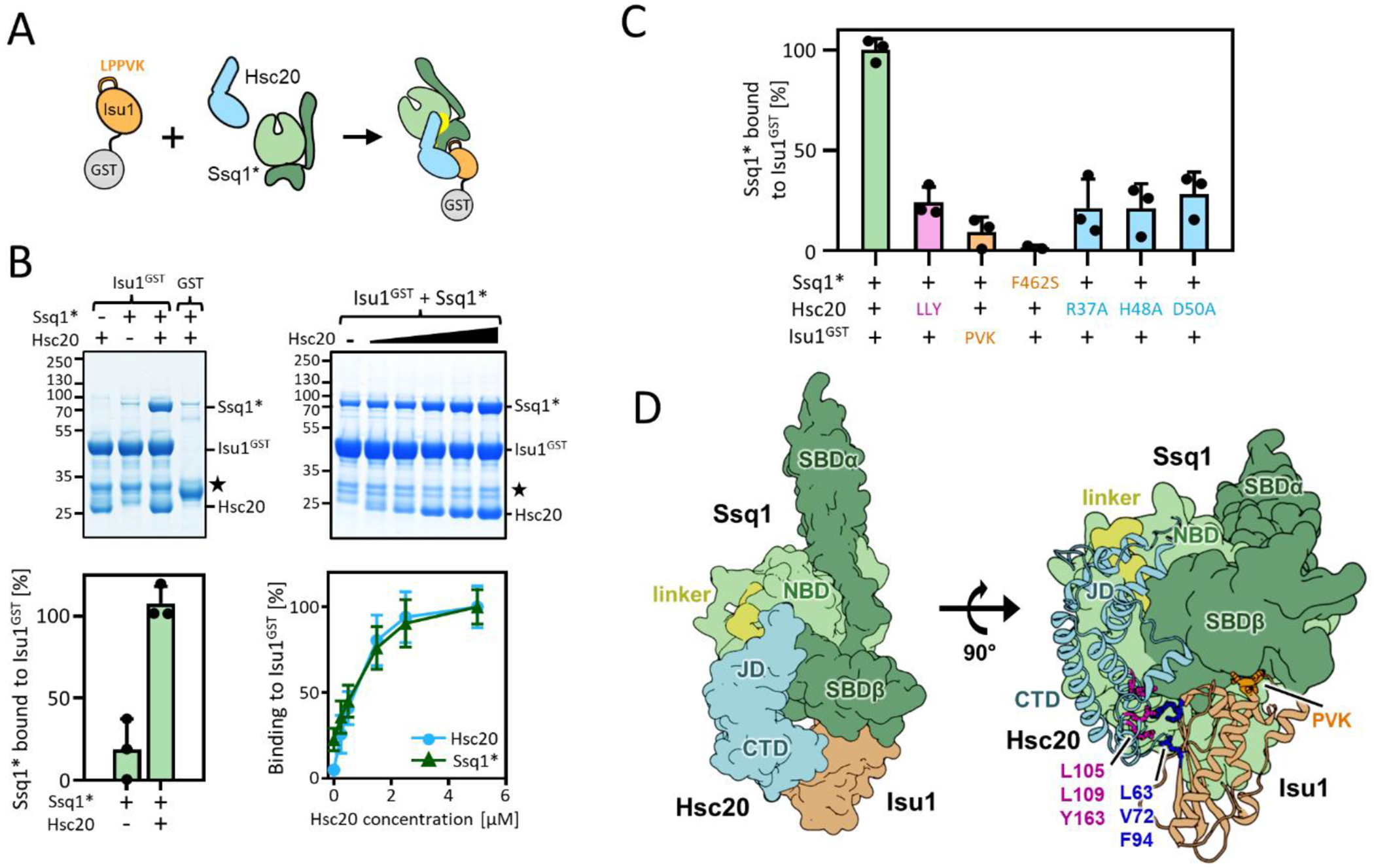
(A-C) To assess tripartite complex formation pulldown assays were performed. Isu1GST (2.5 μM) was incubated in the presence of Hsc20 and/or Ssq1* (2.5 μM and 5 μM, respectively unless otherwise indicated). Glutathione resin was added to pull-down GST and associated proteins, which were then separated by SDS-PAGE and stained with Coomassie blue (entire gels and controls in indicated Supplemental Figures). Molecular weight markers in kDa on left. ★; Isu1GST degradation product. Amounts of pulled down Ssq1 were quantified by densitometry and corrected for background binding to GST alone for three independent experiments. Error bars represent SD. Maximum binding set at 100%. (A) Schematic of basic pulldown assay. (B) Dependence of Ssq1* pulldown on presence of Hsc20. Representative gel at top, quantitation at bottom (for details see Supplmental Materials and Methods). (left) in presence and absence of Hsc20. (right) with increasing concentrations of Hsc20 (0.0, 0.25, 0.5, 1.5, 2.5, 5.0 μM). Entire gels and controls, Figure S2. (C) Disruption of individual protein-protein interactions affects formation of tripartite complex. +, WT protein; see Table 1 for key to residues of indicated substitutions and Figures S3 and S4 for gels. (D) Computational model of the tripartite Ssq1(ATP)-Hsc20-Isu1 complex, generated by docking model of Isu1 to the previously published [32] model of Hsc20-Ssq1 complex and refining the obtained dominant structural state by all-atom MD simulations (8.6 μs). (left) Surface representation shows overall architecture of the complex: Ssq1 in the ATP bound conformation with SBDβ and SBDα (dark green) docked to NBD (light green) and interdomain linker (yellow) placed inside the NBD. Hsc20 (cyan) interacts with Ssq1 via J-domain (JD) at the NBD/SBDβ, linker interface and with Isu1 via C-terminal domain (CTD). (right) cartoon representation of Hsc20 and Isu1 in the tripartite complex show Hsc20-Isu1 and Ssq1-Isu1 interfaces; L63, V72 and F94 of Isu1 in contact with L105, L109 and Y163 of Hsc20 and LPPVK (PVK) of Isu1 in proximity to the substrate binding cleft of SBDβ. (Hsc20-Ssq1 interface, Figure S5(A).
