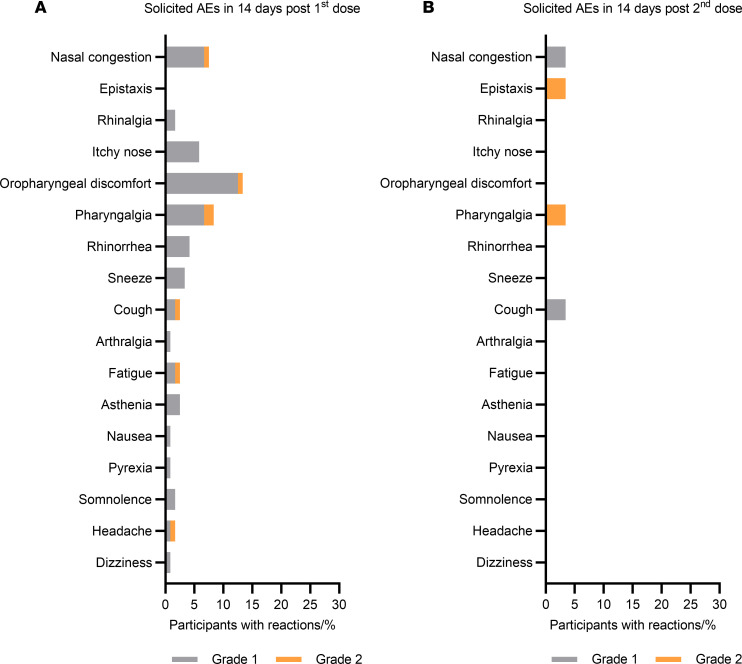
Figure 2. Solicited adverse events following intranasal vaccination.
(A) The solicited local and systemic adverse events (AEs) were reported by each participant within 14 days after the first dose (n = 120). (B) The solicited local AEs were reported by each participant within 14 days after the second dose (n = 29). No systemic AEs were reported within 14 days after the second dose. Gray shading represents grade 1 (mild) events, and orange shading illustrates grade 2 (moderate) events. The AE categories followed the guidelines promulgated by the Center for Drug Evaluation of the National Medical Products Administration.