Extended Data Figure 2. Cryo-EM analysis of the chimeric Sec complex in an apo form.
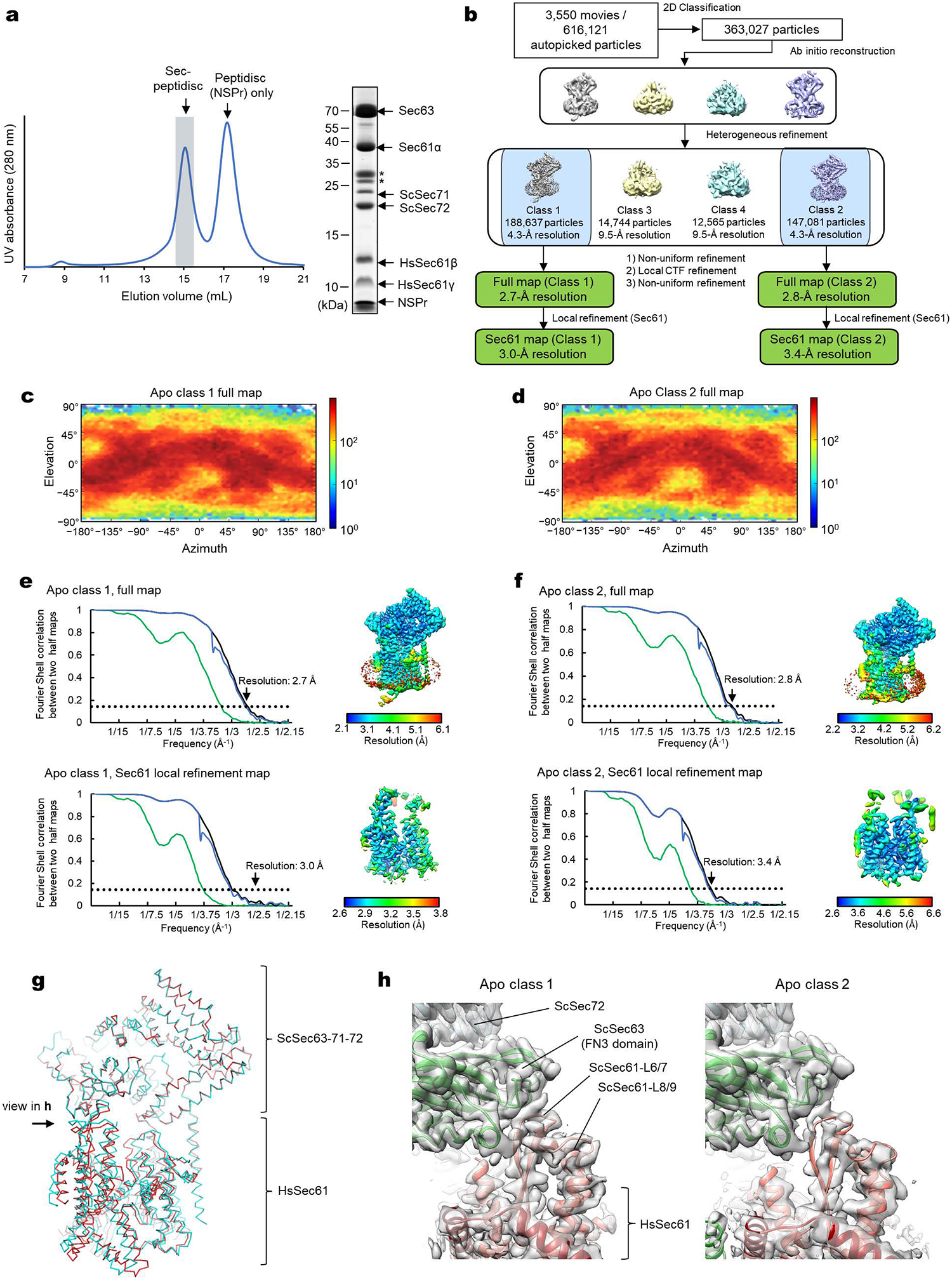
a, Purification of the chimeric Sec complex reconstituted in a peptidisc. Left, Superose 6 elution profile; right, Coomassie-stained SDS gel of the peak fraction. The fraction marked by gray shade was used for cryo-EM. Asterisks, putative species of glycosylated ScSec71. The experiment was repeated at least four times independently with similar results. b, A schematic of the cryo-EM analysis of the chimeric Sec complex in an apo state. c and d, Distributions of particle view orientations in the final reconstructions of Classes 1 (c) and 2 (d). e and f, Fourier shell correlation (FSC) curves and local resolution maps of the final reconstructions. g, Superimposition of the Class 1 and 2 atomic models (based on the cytosolic domains) shows a slight difference in relative positions between Sec63–Sec71–Sec72 and the Sec61 complex. h, Side views showing the contact between the engineered cytosolic loops of Sec61α and the FN3 domain of ScSec63. Note that in Apo Class 2, the contact is more poorly packed than Class 1.
