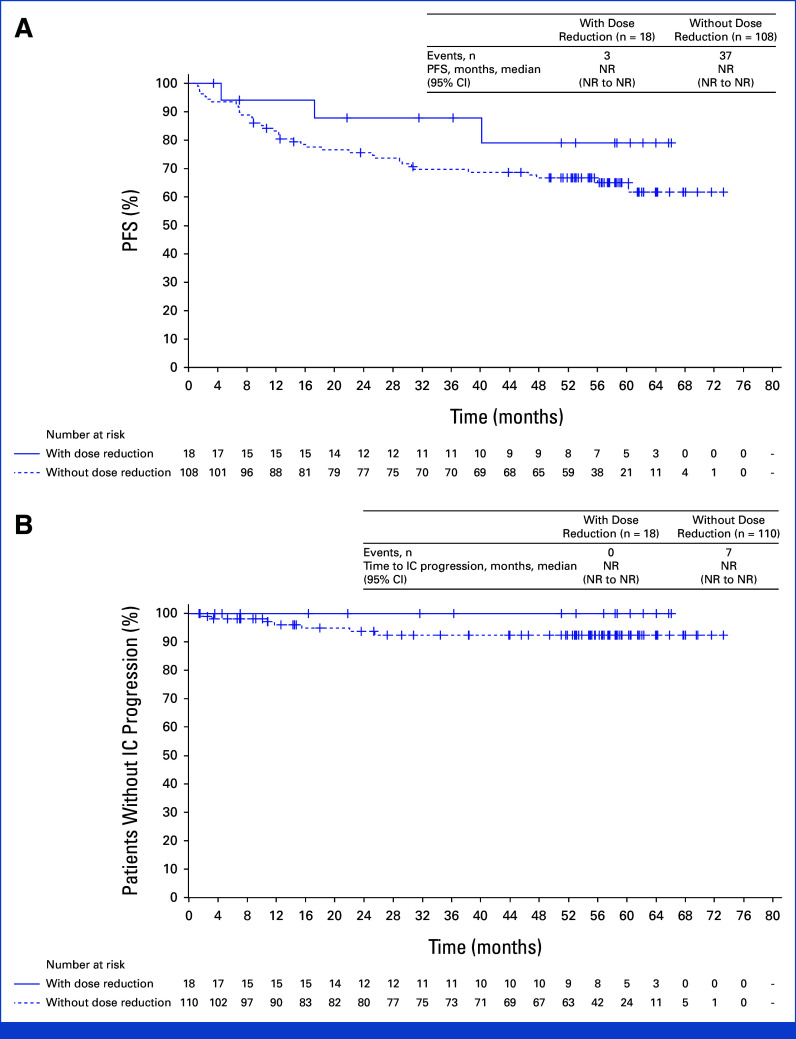
FIG 4.
Outcomes in patients who had first lorlatinib dose reduction within 16 weeks. (A) PFS by investigator assessment and (B) time to intracranial progression by investigator assessment. PFS and time to intracranial progression were recalculated starting at the landmark time. Patients with event/censor time within the landmark time were excluded from the analysis. HR, hazard ratio; IC, intracranial; NR, not reached; PFS, progression-free survival.