Abstract
The rapid rise of antibiotic-resistant strains and the persistence of biofilm-associated infections have significantly challenged global public health. Unfortunately, current clinical high-dose antibiotic regimens and combination therapies often fail to completely eradicate these infections, which can lead to adverse side effects and further drug resistance. Amidst this challenge, however, the burgeoning development in nanotechnology and nanomaterials brings hopes. This review provides a comprehensive summary of recent advancements in nanomaterials for treating bacterial infections. Firstly, the research progress of catalytic therapies in the field of antimicrobials is comprehensively discussed. Thereafter, we systematically discuss the strategies of nanomaterials for anti-bacterial infection therapies, including endogenous response catalytic therapy, exogenous stimulation catalytic therapy, and catalytic immunotherapy, in order to elucidate the mechanism of nanocatalytic anti-infections. Based on the current state of the art, we conclude with insights on the remaining challenges and future prospects in this rapidly emerging field.
Keywords: Nano-catalysis, Antibacterial, Reactive oxygen species, Biomedicine
Graphical abstract

1. Introduction
Bacterial infections pose a significant global public health challenge due to the rise of antibiotic-resistant strains and persistent biofilm-associated infections. [[1], [2], [3], [4]]. The World Health Organization (WHO) identifies antibiotic resistance as a major threat to global health, food security, and development [5]. Pathogens like Methicillin-resistant Staphylococcus aureus (MRSA) and multidrug-resistant (MDR) Gram-negative bacteria have developed resistance to multiple antibiotics, making standard treatments ineffective [[6], [7], [8]]. Additionally, bacteria can form biofilms, structured communities encased in extracellular polymeric substances (EPS), which enhance their resistance to antibiotics [[9], [10], [11]]. The EPS matrix acts as a barrier, preventing antimicrobial agents and immune cells from penetrating, leading to chronic and recurrent infections [12]. Current therapeutic strategies, including high-dose antibiotic regimens and combination therapies, often fail to completely eradicate these infections and may cause adverse side effects and further resistance. Consequently, there is an urgent need for novel antimicrobial strategies to effectively address these complex bacterial infections.
Nanocatalytic therapy (NCT) has emerged as a groundbreaking approach against bacterial infections [[13], [14], [15]]. This strategy employs nanoscale catalysts to generate reactive oxygen species (ROS) and other intermediates to kill bacteria [16]. These nanocatalysts can interact with endogenous H2O2 through mechanisms such as chemical and nanozyme catalysis, or can be activated by external stimuli, including light (photocatalysis), ultrasound (sonocatalysis), and electric fields (electrocatalysis). The ROS produced, including hydroxyl radicals (·OH), superoxide anions (·O2−), and singlet oxygen (1O2), are highly effective at damaging bacterial cell membranes, proteins, and DNA, leading to bacterial cell death [17]. NCT provides advantages over traditional antibiotics by targeting bacteria via oxidative stress, thereby reducing the potential for resistance development. Additionally, the versatility of nanocatalysts allows for precise control over their antimicrobial activity and can be engineered to possess additional functionalities, such as targeted delivery, enhanced permeability, and the ability to modulate the host immune response [18,19]. This multifunctionality makes NCT a versatile and powerful tool in the fight against bacterial infections.
The design and optimization of nanocatalysts are essential for enhancing their therapeutic efficacy [20,21]. Various metal-based nanocatalysts and metal oxides have shown exceptional catalytic properties. Hybrid structures like metal-organic frameworks (MOFs) and covalent organic frameworks (COFs) enable precise customization of catalytic activity and biocompatibility [[22], [23], [24]]. Functionalizing these materials with targeting ligands enhances specificity and minimizes off-target effects. Moreover, integrating nanocatalytic therapy with other treatment modalities, such as photothermal therapy and immunotherapy, can provide synergistic effects, significantly boosting overall antimicrobial efficacy [25,26].
In recent years, antibacterial treatments based on nanocatalytic medicine have garnered increasing research interest and achieved significant advancements. However, this research direction has yet to be comprehensively reviewed and discussed. Herein, we systematically overview and discuss the latest achievements in nanocatalytic medicine within the antibacterial field, providing a thorough review based on representative examples. This article classifies nanocatalytic therapies into endogenous stimulus-mediated (chemodynamic and nanozyme catalytic therapy) and external stimulus-mediated (photocatalytic, sonocatalytic, and electrocatalytic therapy) approaches, emphasizing the mechanisms, design principles, and benefits of each strategy (Fig. 1). Furthermore, we describe the latest applications of nanocatalytic medicine combined with immunotherapy in antibacterial treatment and their immunomodulatory mechanisms. Finally, we discuss the current challenges and clinical application prospects in this field, aiming to provide insights for its future development.
Fig. 1.
Schematic diagram of nanocatalytic therapy for bacterial infections.
2. Endogenous-stimuli-mediated nanocatalytic medicine anti-infection
2.1. Chemodynamic therapy (CDT)
Shi et al. introduced CDT as a therapeutic approach where chemical stimulation induces ROS production in 2016 [27]. In acidic environments, it leverages Fenton and Fenton-like reactions to transform H2O2 into toxic ·OH using Fe2+ or other transition metal ions such as Cu2+, Co2+, Mn2+, achieving therapeutic outcomes without external physical stimulation. The typical reaction equation is as follows (M refers to Fe, Cu, Mn, Co, etc.):
| Mn+ + H2O2 → M(n+1)+ + ·OH + OH− | (1) |
| M(n+1)+ + H2O2 → M(n)+ + ·O2H + H+ | (2) |
CDT is frequently utilized in the development of nanomaterials for cancer therapy due to the mild acidity and elevated H2O2 levels in the tumor microenvironment (TME) [[28], [29], [30], [31]]. Given that the infection microenvironment, particularly the biofilm microenvironment (BME), shares similar characteristics with the tumor microenvironment, namely higher levels of glutathione (GSH) and H2O2 as well as an acidic pH compared to normal tissues, CDT has gained attention as a promising strategy for infection treatment [32]. This approach can induce endogenous chemical reactions at infection sites, generating ROS to eliminate pathogens while sparing normal tissues, thereby enhancing therapeutic specificity and reducing side effects. Li and co-workers synthesized FePS3 nanosheets using Fe, P, and S powders as raw materials through a high-temperature solid-state reaction (Fig. 2A–C) [33]. Under the acidic pH conditions of the infection microenvironment, FePS3 nanosheets release Fe2+, which decomposes H2O2 into toxic ROS via the Fenton reaction, thereby achieving antimicrobial effects. Additionally, the reductive [P2S6]4- can reduce Fe3+ to Fe2+, further enhancing the efficacy of CDT. However, bacteria can protect themselves from the host immune system and antibiotics by forming biofilms. Especially in implant-associated infections (IAIs), biofilm is a major cause of refractory and chronic infection. Therefore, eliminating bacterial biofilms is one of the key goals in anti-infective therapy. Bacterial biofilms are intricate assemblies of bacteria and their secreted EPS, serving as physical and chemical shields for the enclosed bacteria. EPS in biofilm primarily consists of polysaccharides, proteins, and extracellular DNA (eDNA), with eDNA being essential for biofilm formation, maturation, and structural stability. The ·OH produced by CDT, with a redox potential of 2.80 eV, can effectively damage DNA structure. Based on this, Guo et al. proposed a space-selective CDT strategy for treating IAIs. Using iron oleate [Fe(OA)3] and copper oleate [Cu(OA)2] complexes, CuFe5O8 nanocubes (NCs) were synthesized. These CuFe5O8 NCs were shown to generate high levels of ·OH via the Fenton reaction within biofilms, disrupting eDNA and degrading the biofilm, thus compromising its barrier function (Fig. 2D–H) [34]. Outside the biofilm, the CuFe5O8 NCs catalyzed the production of low levels of ·OH, which promoted macrophage polarization toward the M1 phenotype, reversing the immunosuppressive microenvironment of the biofilm.
Fig. 2.
Nanomaterials using CDT strategies to combat bacterial infections. A) The schematic illustration of the strategy and process for constructing FePS3 nanosheets (FePS3 NSs). B) EPR spectra of FePS3 NSs under different pH and concentration conditions in the presence of H2O2. C) FePS3 NSs exhibit self-enhanced Fenton activity in acidic infection environments, generating ROS to eliminate the infection. D) The synthesis process of CuFe5O8 NCs. E) Schematic illustration of CuFe5O8 NCs using CDT strategy to generate ·OH via Fenton reaction to disrupt biofilm structure and modulate immunity. F) 3D reconstructed images of S. aureus and E. coli biofilms after treatment with CuFe5O8 NCs. G) Results of eDNA content in the biofilms of S. aureus and E. coli after treatment with CuFe5O8 NCs. H) Agarose gel electrophoresis images showing DNA damage by ·OH generated through the Fenton reaction with CuFe5O8 NCs. A-C) Reproduced with permission [33]. Copyright 2021, The Royal Society of Chemistry. D-H) Reproduced with permission [34]. Copyright 2020, ACS Publications.
The efficacy of using CDT strategies to treat bacterial infections depends on the activity of the Fenton reaction. The activity of the Fenton reaction is closely related to the concentrations of catalytic ions and H2O2, as well as local pH and temperature conditions. Additionally, excessively high local concentrations of GSH can weaken the bactericidal ability of the ·OH produced by the Fenton reaction. Despite the acidic pH (6.5–7.0) and overexpression of H2O2 at bacterial infection sites compared to normal tissues, these conditions are still insufficient to efficiently initiate the Fenton reaction to produce an adequate amount of ·OH. Excessive GSH production at the infection site can neutralize ·OH, reducing the antimicrobial efficacy of the CDT strategy. In view of this, researchers have adopted various strategies in recent years to enhance the efficacy of CDT. These strategies mainly include increasing the H2O2 concentration, lowering the pH of the reaction condition, raising the local temperature, and reducing the GSH levels in the infection microenvironment. All these approaches have successfully amplified the Fenton reaction and improved the antibacterial efficiency of nanomaterials. Li and co-workers developed a polyetheretherketone (PEEK) implant incorporating copper sulfide/graphene oxide (CuS/GO) bio-heterojunctions (bioHJs) and glucose oxidase (GOx) for treating diabetic wound infections (Fig. 3A and B) [35]. In the diabetic infectious microenvironment (DIM), GOx catalyzes the conversion of glucose into H2O2, initiating the reaction. Subsequently, Cu2+ released from bioHJs decomposes H2O2 into highly toxic ·OH through the Fenton reaction, serving as the second step of the reaction. Additionally, this PEEK material exhibits excellent photothermal and photodynamic properties, capable of generating abundant singlet oxygen (1O2) and superoxide (·O2−) under laser irradiation. This dual-engine strategy has been shown to enhance the Fenton reaction, producing numerous toxic ROS that can cause bacterial cell membrane rupture and intracellular content leakage, ultimately leading to bacterial death. Sun et al. utilized MIL-101 (Fe)-derived cascade nano-enzymes to kill multidrug-resistant bacteria and accelerate the healing of infected wounds by enhancing CDT strategy (Fig. 3C–F) [36]. The engineered FeS2@MoS2 nanocubes exhibit POD-, CAT-, SOD-like activities, and the ability to deplete GSH. In the acidic infection microenvironment, the FeS2@MoS2 NPS exhibit SOD-like activities, promoting the conversion of superoxide anions (·O2−) into H2O2. Subsequently, the generated H2O2 serves as a substrate for the Fenton reaction with the FeS2@MoS2 NPS, producing ·OH radicals capable of killing bacteria. FeS2@MoS2 NPS can lower GSH levels at infection sites and catalyze nicotinamide adenine dinucleotide phosphate (NADPH) decomposition, inhibiting glutathione regeneration and enhancing the antimicrobial efficacy of ·OH radicals. Owing to the multiple nanozyme activities of FeS2@MoS2 NPS, which effectively eliminate MDR E. coli and MRSA infections, this nanomaterial provides a strategy for designing cascade nanozyme platforms combined with CDT for the treatment of bacterial infections.
Fig. 3.
Enhancing CDT strategies to amplify the Fenton reaction and improve the antibacterial efficacy of nanomaterials. A) Schematic of the CuS/GO bioHJs-engineered and GOx-coated PEEK implant synthesis. B) Mechanism by which CuS/GO bioHJs-engineered and GOx-coated PEEK boosts the Fenton reaction and enhances bactericidal efficiency in infection microenvironments by increasing H₂O₂ concentration, lowering GSH levels, and raising reaction temperature. C) Synthesis of the FeS₂@MoS₂ cascade nano-system. D) FeS₂@MoS₂ NPs enhance the Fenton reaction to combat multidrug-resistant bacteria and aid infected wound healing. E) Schematic of FeS₂@MoS₂ nanozymes with POD-, CAT-, and SOD-like activities and their catalytic mechanisms. F) Live/dead staining and SEM images of bacteria after FeS₂@MoS₂ nanozyme treatment. A-B) Reproduced with permission [35]. Copyright 2022, Wiley-VCH. C-F) Reproduced with permission [36]. Copyright 2024, Wiley-VCH.
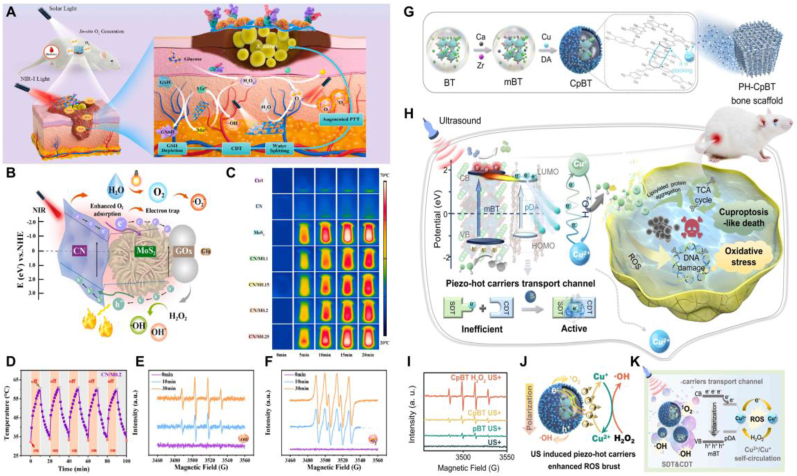
Given that antimicrobial therapy using CDT strategies alone is sometimes not therapeutically effective, more researchers have recently begun employing combined treatment strategies based on CDT to address bacterial infection challenges. Localized high temperatures have been shown to enhance the Fenton reaction, making the combination of CDT and photothermal therapy (PTT) an ideal synergistic strategy for antibacterial treatment. Zhang et al. created a two-stage nanosheet-structured iron hydroxide composite coating on Mg alloy, followed by thermal reduction to disrupt surface Fe-OH bonds [37]. This process improved photothermal conversion efficiency and increased Fe2+ content, enhancing Fenton reaction activity. Consequently, this multifunctional Mg-based implant coating material exhibited synergistic photothermal/chemodynamic antibacterial efficacy against bacterial infections. Considering the deep tissue penetration advantage of NIR-II laser, He et al. attached Pt nanoparticles (Pt NPs) to V2C MXene to form an artificial nanoplatform (Pt@V2C) [38]. They demonstrated that under NIR-II laser irradiation, Pt@V2C exhibited enhanced CDT activity and photothermal activity, effectively eliminating methicillin-resistant Staphylococcus aureus (MRSA) from deep tissues within subcutaneous abscesses and bacterial keratitis. Through the synergistic action of PTT/CDT, this approach accelerated abscess resolution and promoted wound and corneal healing. Deng et al. designed and developed a photoactivated cascaded bio-heterojunction, which under dual-light conditions can generate ·OH and ·O2− through CDT synergizing with PDT for anti-infective therapy, while the material also exhibits good photothermal performance (Fig. 4A–F) [39]. They elucidated the excellent therapeutic effects of a CDT-based therapy combined with PDT/PTT on diabetic bacterial wound infections. In addition, Liu et al. innovatively composed a nanocomplex of polydopamine/hemoglobin/Fe2+ and loaded it with antimicrobial peptides, named PL@HPFTM nanoparticles [40]. These nanoparticles catalyze the generation of ·OH through the reaction between Fe2+ and H2O2 at the infection site, while the efficient photothermal conversion increases the temperature and the sustained release of antimicrobial peptides. This combined therapy strategy of CDT/PTT/antimicrobial peptides significantly enhances the bactericidal efficiency, eliminating bacterial infections. Sonodynamic therapy (SDT) is another treatment strategy that achieves therapeutic effects by generating ROS, showing excellent efficacy against bacterial infections as well. Huang et al. developed a multifunctional nanoreactor by integrating piezoelectric barium titanate with polydopamine and copper, utilizing spatiotemporal ultrasound-driven tandem catalysis to enhance sonodynamic and chemical dynamic therapy efficacy (Fig. 4G–K) [41]. This combined CDT and SDT strategy produces substantial ROS, disrupting bacterial membrane structure, damaging DNA, and inducing cuproptosis-like bacterial death. This strategy exhibits excellent antibacterial performance under ultrasound stimulation, significantly reducing IAIs caused by bacterial biofilm formation. CDT and CDT-based combination therapies have demonstrated efficacy against bacterial infections, including those caused by drug-resistant strains. In the future, more nanocatalytic platforms based on CDT strategies are expected to be designed and developed, further enhancing the efficiency of antimicrobial treatments and reducing associated adverse effects.
Fig. 4.
CDT-based combined therapeutic strategies to tackle bacterial infection challenges. A) C-bio-HJs nanomaterials improve CDT strategies when combined with PDT and PTT therapies, helping to eliminate bacterial infections and promote the healing of diabetic wounds. B) Schematic representation of the electron transfer process between CN and MoS₂ based on DFT calculations, illustrating the synergistic antibacterial mechanism of C-bio-HJs. C-D) Real-time photothermal images and cycling curves of nanomaterials under continuous laser irradiation. E-F) The ability of nanomaterials to generate ·OH and ·O₂⁻ under dual light irradiation. G-H) Schematic of the CpBT nanoreactor preparation process and its ability to trigger SDT and CDT cascade reactions under US activation to treat S. aureus infections. I-K) EPR spectra of ·OH generated by CpBT nanoreactors and a schematic of CpBT nanoreactors enhancing CDT strategies under US stimulation. A-F) Reproduced with permission [39]. Copyright 2022, Elsevier Ltd. G-K) Reproduced with permission [41]. Copyright 2024, Springer Nature.
Chemodynamic therapy for bacterial infections is an effective and promising treatment approach. Although it depends on the chemical conditions of the specific microenvironment, researchers are also focused on optimizing material design, reducing chemical toxicity and improving antibacterial efficiency based on chemokinetic therapy [42]. Additionally, combination therapy based on CDT is emerging as a key direction and trend in the future of nanocatalytic medical treatments for bacterial infections [38].
2.2. Nanozyme catalytic therapy (NCT)
Nanozymes are artificial enzymes based on nanomaterials, first proposed by Scrimin and colleagues [43]. Nanozyme catalytic therapy is a method that uses nanomaterials with enzyme-like activities to treat diseases. These nanomaterials are typically designed to exhibit activities similar to natural enzymes, such as POD-like activity, CAT-like activity, and SOD -like activity. Enzyme catalysis is one of the most common catalytic reactions that maintain physiological homeostasis of biological systems [44]. Since the substrates of enzyme catalysis are biomolecules, these reactions typically occur under mild physical conditions. Despite natural enzymes having high substrate selectivity and catalytic efficiencies superior to synthetic catalysts, they are limited by high production costs, low yields, and susceptibility to denaturation and inactivation during transportation and storage, which restrict their applications in biological contexts [45,46]. As an alternative, nanozymes, composed of materials such as metal-organic compounds, inorganic crystals, or organic polymers, offer high stability and sustained enzyme reactions [47]. In the past, therapeutic strategies involving nanozyme-catalyzed ROS generation have been extensively studied in cancer treatment, yielding excellent therapeutic outcomes [[48], [49], [50], [51]]. Lately, the application of nano-enzyme catalyzed therapy in the treatment of bacterial infections has gained attention and is showing promising potential [[52], [53], [54]]. Different types of nanozymes possess distinct physicochemical properties and structures, resulting in varied antimicrobial efficacy.
Metal-based nanozymes, often incorporating noble metals like platinum, silver, and gold, demonstrate significant catalytic activity and are used in antimicrobial applications [55,56]. Hu and co-workers grew ultrasmall Au nanoparticles (UsAuNPs) by in situ reduction on ultrathin 2D MOFs, and prepared UsAuNPs/MOFs hybrids [57]. These hybrids leverage the benefits of both UsAuNPs and ultrathin 2D MOFs, showing excellent POD-like activity capable of decomposing H2O2 into toxic ·OH. The metal-based nanozyme material exhibits excellent antibacterial properties against both E. coli as well as S. aureus in vitro and in vivo, promotes wound healing, and is biocompatible. Yao et al. designed and synthesized dual-metal BiPt nanozymes coated with platelet-bacteria hybrid membrane (BiPt@HMVs) [58]. The synthesized dual-metal nanozymes exhibited dual enzyme-like activities, including peroxidase and oxidase mimic activities, and ultrasound stimulation greatly enhanced their catalytic efficiency. The platelet-bacteria hybrid membrane coating on the nanozymes endowed the material with excellent infection site targeting ability and showed outstanding therapeutic effects against infections caused by clinically relevant bacteria. To address the complex requirements of antibacterial therapy that cannot be met by nanozyme models with single enzyme activity, Shan et al. designed and developed nanozymes with multiple enzyme activities and synthesized a novel metal enzyme (Ru-PC NPs) (Fig. 5A–B) [59]. Under acidic conditions, the synthesized Ru-PC NPs can consume GSH at the infection site and catalyze the production of ·OH through POD-like activity for antibacterial therapy. Under neutral conditions, Ru-PC NPs display CAT-like activity, breaking down H₂O₂ to produce O₂, which helps alleviate infection-related hypoxia. Additionally, its SOD-like activity eliminates excess ROS in neutral environments, preventing excessive inflammation. The quadruple enzyme activity of the synthesized Ru-PC NPs highlights its significant potential in antibacterial therapy and wound healing promotion.
Fig. 5.
Metal-based nanozymes and metal oxide/sulfide-based nanozymes are used for anti-infective therapy. A) Diagram of the synthesis process of nanozymes (Ru-PC NPs). B) The nanozyme (Ru-PC NPs) exhibits quadruple enzyme-like activities under acidic and neutral conditions. Under acidic conditions, it shows POD-like activity, consuming GSH and catalyzing the generation of ·OH from H2O2. Under neutral conditions, it exhibits CAT-like and SOD-like activities, catalyzing the decomposition of H2O2 to produce O2 and clearing excess ROS. C) Diagram of the synthesis process of CuCo2O4 nanoflowers. D) Schematic illustration of CuCo2O4 nanoflowers eradicating pathogenic biofilms. E) Mechanism of CuCo2O4 nanoflowers generating abundant ROS through a multi-enzyme catalytic platform to combat bacterial infections. A-B) Reproduced with permission [59]. Copyright 2024, Elsevier Ltd. C-E) Reproduced with permission [66]. Copyright 2024, ACS Publications.
Metal oxide/sulfide-based nanozymes have shown remarkable enzyme-like catalytic activity, and several studies have highlighted their use as nanozyme catalysts in antibacterial research [[60], [61], [62], [63]]. Li et al. synthesized tannic acid-chelated Fe-modified MoS2 nanosheets (MoS2@TA/Fe NSs) and integrated them into a multifunctional hydrogel, imparting it with POD and CAT-like activities [64]. Under acidic conditions, MoS2 in the nanozyme system catalyzes the production of toxic ·OH from H2O2 via POD-like activity, while under neutral conditions, TA/Fe in the nanozyme system catalyzes the generation of O2 from H2O2 via CAT-like activity, relieving hypoxia at the infection site. Furthermore, MoS2@TA/Fe NSs exhibit excellent antioxidant capabilities, capable of eliminating excessive ROS generated at the infected wound, thus accelerating the wound healing process. Liu et al. fabricated multi-enzyme-like nanozymes by decorating manganese oxide nanoclusters on graphdiyne nanosheets (MnOx/GDY) and embedded them in hyaluronic acid and polymethyl methacrylate-based microneedles (MGMN) [65]. The prepared multi-enzyme catalytic platform possesses OXD, POD, CAT, and SOD-like activities, and also exhibits antibacterial and anti-inflammatory effects. They demonstrated that this multifunctional enzymatic nano-catalytic platform can eradicate pathogens, prevent biofilm formation, alleviate corneal inflammation, relieve ocular hypoxia, and accelerate the repair of corneal damage. Wang and co-workers synthesized CuCo2O4 nanoflowers, which possess dual enzyme activities of POD-like and OXD-like activities, capable of catalyzing the production of toxic ·OH and ·O2− from H2O2 (Fig. 5C–E) [66]. Additionally, CuCo2O4 nanoflowers exhibit glutathione peroxidase-like (GSH-Px-like) activity, which can degrade GSH at the infection site, further enhancing the ROS-mediated killing efficiency against infecting bacteria. They demonstrated that the CuCo2O4 nanoflowers, through its excellent enzymatic catalytic activities, can disrupt bacterial cell membrane structures, ultimately leading to copper-induced bacterial death, providing new insights and inspiration for treating bacterial biofilm infections.
MOFs are porous materials composed of metal ions or clusters coordinated with organic ligands. Their highly customizable structures and functional characteristics have attracted significant attention in biomedical applications [67]. The excellent physicochemical properties of MOF materials provide several advantages to MOF-based nanozyme catalytic platforms compared to traditional nanozymes [[68], [69], [70], [71]]: (i) The porous structure and large surface area of MOFs allow substrates to fully reach the active sites, effectively increasing the catalytic reaction rate. (ii) The structure of MOFs is tunable, and their surfaces and pores can be functionalized to optimize catalytic performance. (iii) MOF-based nanozymes are capable of displaying multiple enzyme activities simultaneously (including oxidase, peroxidase, and catalase), allowing for multifunctional catalysis in complex biological environments. (iv) MOFs possess good chemical stability, maintaining their structure and function in various extreme environments. More importantly, MOF-based biomaterials generally exhibit good biocompatibility, which enables their safe use in vivo without causing significant toxicity, demonstrating promising potential for antimicrobial applications [72,73]. Wang and colleagues introduced an acid-enhanced dual-mode antibacterial strategy using zeolitic imidazolate framework-8 (ZIF-8)-derived nanozymes, incorporating glucose oxidase (GOx) and gold nanoparticles (Au NPs) into ZIF-8. This approach enables ROS production through cascade catalytic reactions within the infection microenvironment [74]. This MOF-based nanocatalytic platform exhibited excellent bactericidal effects against bacteria at low doses (4–8 μg/mL), demonstrating the great potential of MOF-derived nanozymes for antibacterial applications. Wu et al. innovatively doped PCN-222 MOFs with bismuth nanoparticles (Bi NPs) to synthesize a biomimetic nanozyme catalyst, Bi-PCN-222 (Fig. 6A–E) [75]. When bacteria come into contact with Bi-PCN-222 in a physiological environment, its optimal redox potential triggers electron transfer via the bacterial membrane's electron transport pathways, ultimately penetrating the bacteria and disrupting their respiration and metabolism, resulting in the elimination of over 99.9 % of S. aureus. Furthermore, Liu et al. prepared hybrid nanozymes (Fe3O4@MOF@Au NPs, FMA NPs) by in situ growth of ultrasmall Au NPs on MOF- stabilised Fe3O4 NPs [76]. The FMA NPs demonstrated increased POD-like activity, efficiently catalyzing the generation of toxic ·OH in the presence of a low concentration of H₂O₂ (0.97 mM). They also demonstrated the strong bactericidal effects of FMA NPs against pathogens at the infection site while displaying excellent biocompatibility.
Fig. 6.
MOF-based nanozymes and single-atom nanozymes for antibacterial therapy. A) Schematic representation of the antibacterial mechanism of the biomimetic nanozyme catalyst, Bi-PCN-222. B) HRTEM images and elemental mapping of Bi-PCN-222 nanozymes. C-E) DFT simulation results for Bi-PCN-222 nanozymes. F) Synthesis procedure of Fe-SAC. G-H) Schematic representation of Fe-SAC's antibacterial mechanism. I) TEM images and EDS elemental mapping of Fe-SAC. J) High-resolution HAADF-STEM image of Fe-SAC. A-E) Reproduced with permission [75]. Copyright 2023, ACS Publications. F-J) Reproduced with permission [78]. Copyright 2024, Wiley-VCH.
Single-atom nanozymes (SAzymes) are a novel class of artificial enzymes composed of individual metal atoms dispersed on a nanomaterial substrate. These single atoms are anchored at specific sites through strong interactions with the substrate material, forming nanostructures with unique catalytic activities [77,78]. Given the numerous advantages of SAzymes, including high catalytic activity, high selectivity, and high stability, making it an excellent alternative to natural enzymes and a research hotspot in nanozyme catalytic strategies [[79], [80], [81]]. Owing to the excellent catalytic activity of SAzymes, using SAzymes for antimicrobial therapy has gradually attracted the interest of researchers. Bai et al. presented a strategy involving a copper and silk fibroin (Cu-SF) composite for synthesizing copper SAzymes with atomically dispersed copper sites [82]. The synthesized Cu SAzymes exhibit three enzyme activities, including POD-like, CAT-like, and OXD-like activities, efficiently killing bacteria through cascade reactions that generate a large amount of toxic ROS. The data indicate that the synthesized Cu SAzymes effectively combat multidrug-resistant bacteria and eradicate biofilms, showing excellent therapeutic effects on IAIs. Gao et al. prepared Mn SAzymes-functionalized 3D-printed bioceramic scaffolds, which efficiently catalyze the local H2O2 through cascade reactions to produce sufficient ·OH and ·O2− for antibacterial therapy [83]. This study provides an effective approach for the treatment of clinical infectious bone defects. Li et al. synthesized a titanium carbide MXene (Ti₃C₂)-based hybrid incorporating Pt single atoms (Pt-Ti₃C₂) [84]. The Pt single atoms exhibited excellent POD-like activity, catalyzing the in situ conversion of H₂O₂ in the microenvironment into ·OH, effectively combating bacterial infections and eradicating biofilms. This work offers a novel approach for bio-catalytic antibacterial therapy. Most conventional nanozyme technologies encounter major challenges related to size, composition, and the limited availability of natural active sites. To address these issues, Liu and co-workers synthesized iron single-atom structures (Fe-SAC) through nitrogen-doped carbon (Fe-N) and demonstrated a high loading capacity (Fig. 6F–J) [78]. They proved that the synthesized Fe-SAC generates ROS through enzymatic catalytic activity, disrupting bacterial cell membrane structures, eradicating MRSA, and accelerating the wound healing process.
As an endogenous catalytic therapy, nanozyme catalytic therapy offers the advantage of not relying on external energy input, making it more broadly applicable in the field of anti-infection treatment. Compared to traditional antibiotic therapies, it provides higher bactericidal efficiency, lower rates of resistance development, and broad-spectrum antibacterial activity. However, nanozyme catalytic therapy also faces challenges, particularly regarding its distribution within the host, long-term biosafety and metabolism in vivo, which require further investigation. Future research should focus on improving the design of nanozyme catalysts to enhance their catalytic efficiency and biocompatibility, as well as exploring their combination with other therapies to achieve broader antibacterial efficacy.
3. External stimuli-responsive nanocatalytic medicine anti-infection
3.1. Photocatalytic therapy (PCT)
Photocatalytic reactions are essentially light-triggered redox reactions [85]. Under irradiation of specific wavelengths, photocatalytic materials can excite electron transitions to form electron-hole pairs. These electrons and holes react with water molecules and oxygen to generate various ROS and can damage bacterial cell membranes, DNA, and proteins, leading to bacterial death [86]. The reaction equations for the photocatalyst are as follows:
| (3) |
| (4) |
Equation (3) describes a Type I photocatalytic reaction, where light of specific wavelengths activates the photocatalyst to generate electron-hole pairs. These electron-hole pairs can react with O2 and H2O to produce ·O2− and ·OH, respectively. Equation (4) describes a Type II photocatalytic reaction, where in the presence of the photocatalyst and specific wavelengths of light, environmental O2 can be converted into highly toxic 1O2 through direct energy transfer [87]. Photocatalysts can be categorized into Type I and Type II materials based on the type of photocatalytic reactions. Type I photocatalytic materials are mainly semiconductors, including metal oxides, nitrides, carbides, and sulfides. Type II photocatalytic materials predominantly consist of phthalocyanines, porphyrins, black phosphorus, and MOFs [88]. Photocatalytic therapy can generate abundant toxic ROS, and it has been extensively explored and studied in both tumor treatment and antibacterial applications [[89], [90], [91], [92]].
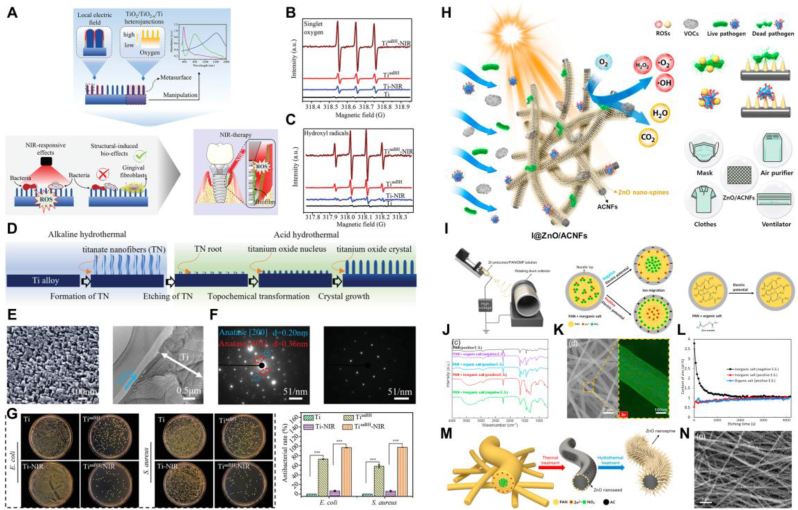
Type I photocatalytic materials generate ·OH and ·O2− through photocatalytic reactions when irradiated with light of energy, which has a strong bactericidal effect. Among type I photocatalytic materials, titanium oxide (TiO2) and zinc oxide (ZnO) nanomaterials have become focal points of research in photocatalytic antibacterial materials. Yang et al. reported a TiO2/TiO2−x metasurface with strong NIR-responsive antibacterial activity on the surface of titanium implants through a novel alkali-acid bidirectional hydrothermal method (aaBH) based on topochemical conversion (Fig. 7A–G) [93]. After low-power NIR irradiation, the material exhibited powerful antibacterial capabilities and excellent biocompatibility. In vivo data showed that the antibacterial efficiency reached 91.80 % after 10 min of NIR laser irradiation, demonstrating the potential of this TiO2/TiO2−x metasurface material for the treatment of IAIs. Kang and co-workers developed a new technique capable of inducing the phase separation of inorganic salts during the electrospinning process to synthesize nanofibers with a surface concentrated in Zn components, named I@ZnO/ACNFs (Fig. 7H–N) [94]. These nanofibers are densely coated with high-aspect-ratio ZnO nanospikes, capable of producing significant amounts of ·OH and ·O₂⁻ through photocatalytic reactions under sunlight. This shows significant antibacterial efficacy against airborne infectious pathogens, demonstrating the potential of photocatalytic materials for disinfection of airborne microorganisms and air purification.
Fig. 7.
Photocatalytic antibacterial strategy based on Type I photocatalysts. A) Preparation process of TiO2/TiO2−x super surfaces. B-C) ESR curves of 1O2 and •OH generated by TiaaBH samples under 808 nm laser irradiation. D) Design principle of constructing quasi-periodic titanium oxide super surfaces on titanium alloy implants. E) SEM and TEM images of TiaaBH. F) Diffraction patterns of TiaaBH and Ti. G) Plate images and corresponding bactericidal rates after different treatments. H) Schematic illustration of the synthesis of ZnO nanospikes grown on activated carbon nanofibers and its photocatalytic antibacterial mechanism. I) Schematic diagram of the electrospinning preparation process and the distribution of inorganic and organic phases under different potentials. J) Infrared spectra of different electrospun fibers. K) TEM and Zn elemental mapping images of electrospun fibers. L) The relationship between atomic content of Zn in the fibers and etching depth. M) Schematic representation of the I@ZnO/ACNFs synthesis process. N) FE-SEM images of I@ZnO/ACNFs. A-G) Reproduced with permission [93]. Copyright 2021, Wiley-VCH. H-N) Reproduced with permission [94]. Copyright 2021, Elsevier Ltd.
Despite Type I photocatalytic materials can produce ·OH and ·O₂⁻ to combat bacterial infections, challenges remain in enhancing their photocatalytic performance. Type I antibacterial photocatalysts, like TiO₂, are wide bandgap semiconductors that can only absorb and utilize ultraviolet (UV) light, which makes up a small fraction of sunlight. Moreover, UV light poses risks to human health and has limited penetration ability [95]. Achieving satisfactory antibacterial effects requires higher light intensity or prolonged exposure, which can significantly raise the toxicity risk to the host [96,97]. Thus, it is essential to broaden the light absorption range, enhance visible light utilization, and boost photocatalytic activity. A practical approach is to modify impurity energy levels by metal and non-metal doping and introducing vacancies, thereby lowering the energy needed to excite photogenerated carriers, expanding the light absorption range, and improving light absorption capacity [98,99]. Lu et al. prepared novel carbon-modified TiO2 nanoparticles (HT) and uniformly dispersed them on reduced graphene oxide (rGO), doping Zn during the preparation process to ultimately obtain the Zn-HT/rGO (HTGZ) nano-system (Fig. 8A–C) [100]. Zn ions were integrated into the TiO₂ nanoparticle lattice, generating additional defect energy levels. The photoexcited electrons could transition from the valence band to the defect levels or from the defect levels to the conduction band, reducing the required photon energy and broadening the optical response range. They proved that the formed HTGZ nano-system significantly increased ROS production under visible light irradiation, enhancing antibacterial performance. Similarly, the photodynamic antibacterial activity of conventional ZnO, with a wide bandgap (∼3.37 eV), relies primarily on ultraviolet light. However, this not only poses risks to normal tissues but also suffers from limitations like low photocatalytic efficiency and poor penetration depth, restricting its use in photocatalytic therapy [101]. To address these challenges, Weng et al. introduced carbonized moxa to synthesize narrow bandgap ZnO (carbonized moxa@ZnO, CMZ) (Fig. 8D–G) [102]. This material exhibits excellent photocatalytic performance, high ROS production, and strong antibacterial efficiency under long-wavelength visible light (yellow light) irradiation. Type I photocatalytic materials like TiO₂ can be combined with other substances, such as MXenes, to enhance electron transfer. Due to their lower energy levels compared to the conduction band of semiconductors and their excellent conductivity, these materials can transfer photogenerated electrons, enabling the separation of electrons and holes, which boosts photocatalytic efficiency. Furthermore, the formation of Schottky junctions prevents the electrons from returning to TiO₂ and recombining with holes. Based on this, Cheng and co-workers prepared ZnTCPP/Ti3C2TX nano-photocatalytic materials, which exhibited excellent antibacterial effects against S. aureus and E. coli under visible light irradiation [103]. After 10 min of visible light irradiation, this material exhibited antibacterial efficiencies of 99.86 % against S. aureus and 99.92 % against E. coli, demonstrating significant potential for enhancing the application of photocatalytic therapy in antibacterial treatments.
Fig. 8.
Photocatalytic enhancement strategy based on Type I photocatalytic materials. A) Schematic illustration of the HTGZ nanosystem enhancing photodynamic antibacterial efficacy and accelerating infected wound healing. B) HR-TEM images of HT, HTG, and HTGZ nanomaterials. C) Plate images of bacteria after intervention with nanomaterials. D) Schematic illustration of the CMZ antibacterial platform producing ROS through photocatalysis and sonocatalysis to eliminate fungi. E) workflow of the in vivo experiment of CMZ against Candida albicans. F) Images of Candida albicans infection sites. G) Area curve of Candida albicans infection sites. A-C) Reproduced with permission [100]. Copyright 2023, Springer Nature. D-G) Reproduced with permission [102]. Copyright 2024, ACS Publications.
When Type II photocatalysts are excited by photons, they absorb energy from the ground state and transition to the excited state, transferring this energy to oxygen molecules. The triplet oxygen then absorbs the energy from the photosensitizer and converts into singlet oxygen (1O₂). While the oxidizing power of 1O₂ is slightly weaker than ·OH, it still exhibits strong antibacterial effects. Therefore, there have been numerous studies on using Type II photocatalysts for antimicrobial treatments based on photocatalytic therapy [104,105]. Zhu et al. developed new AIE photocatalysts by modifying two cationic 4-vinylpyridine units on tetraphenylethylene (TPE) and isolated pure stereoisomers to obtain (Z)-TPE-EPy and (E)-TPE-EPy (Fig. 9A–C) [106]. The synthesized AIE photocatalysts exhibited extremely strong photocatalytic capabilities, producing large amounts of 1O2 and ·O2− after 10 min of white light irradiation. This led to nearly 100 % killing efficiency against Saccharomyces cerevisiae and Candida albicans. In another study, Lochenie and co-workers reported the design of a novel benzothiadiazole photocatalyst with broad-spectrum antibacterial activity under non-toxic light irradiation [107]. They demonstrated that it has significant bactericidal effects against MDR bacteria and it showed excellent therapeutic effects in a pig keratitis model. Black phosphorus (BP) is also an excellent Type II photocatalytic material that can generate a variety of ROS under laser irradiation, making it one of the promising nanomaterials for photocatalytic antibacterial therapy [108]. Based on this, Li et al. developed a mussel-inspired multifunctional photo crosslinked gelatin (GD) hydrogel coating on bioinert PPENK, which contains black phosphorus nanosheets wrapped in PDA (Fig. 9D–E) [109]. Under 808 nm laser irradiation, this coating material can generate and release toxic ROS to achieve antibacterial effects and exhibits good biocompatibility.
Fig. 9.
Photocatalytic antibacterial strategy based on Type II photocatalysts. A) Schematic representation of the structures and antifungal mechanisms of AIE photocatalysts. B) ESR spectra of AIE photocatalysts showing the generation of 1O₂ and ·O₂⁻. C) Energy level diagram depicting the singlet and triplet states of AIE photocatalysts. D) Schematic illustration of the preparation of GelMA/DMA nanocomposite photo-crosslinked hydrogel coating on PPENK implants. E) SEM images of bacteria after treatment with different materials under dark and laser conditions. A-C) Reproduced with permission [106]. Copyright 2022, Springer Nature. D-E) Reproduced with permission [109]. Copyright 2023, Elsevier Ltd.
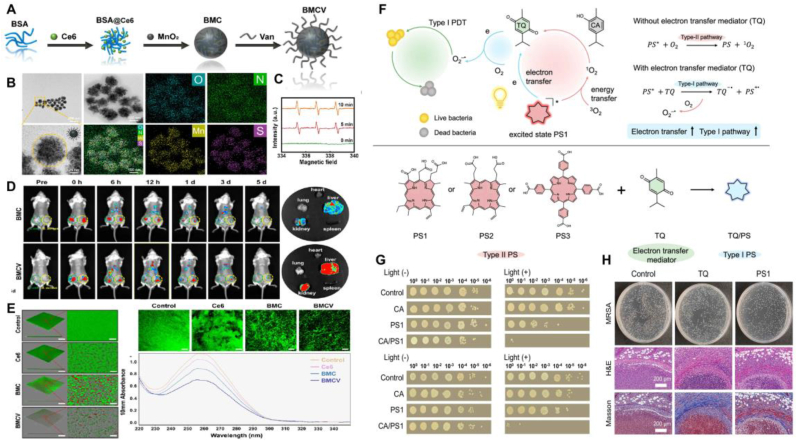
Photocatalytic antibacterial materials based on Type II photocatalytic reactions require O2 to produce 1O2. However, similar to tumor sites, infection sites are often hypoxic environments with insufficient oxygen content, which significantly limits the in vivo antibacterial efficiency of Type II photocatalytic materials. To address this challenge, researchers have investigated several strategies to boost oxygen levels at infection sites, thereby improving the antibacterial effectiveness of photocatalysis. Given the excessive production of H2O2 at infection sites, Wang et al. utilized the catalase-like activity of MnO2 to design and prepare PEG-modified MnO2 nanoparticles loaded with the photosensitizer Ce6 (Ce6@MnO2-PEG NPs) [110]. These nanoparticles can catalyze the endogenous H2O2 to generate O2, thus enhancing the photocatalytic ability of the nanoparticles. They proved that the prepared nanoparticles could efficiently kill S. aureus under laser irradiation, significantly improving the condition of S. aureus-induced mice subcutaneous abscesses. Moreover, reducing the distance between the photosensitizer and the bacteria maximizes the bactericidal effect of photocatalytically produced ROS, making bacterial targeting strategies essential in the design of photocatalytic therapies. Our group designed and developed multifunctional photocatalytic nanoparticles (BMCV NPs) by conjugating vancomycin groups onto the surface of BSA-MnO2 nanoclusters loaded with Ce6, thereby targeting S. aureus (Fig. 10A–E) [111]. The synthesized BMCV NPs can specifically adhere to the surface of S. aureus, catalyze H2O2 to generate O2, and produce ROS through photocatalysis around the bacteria, maximizing the bactericidal effect on S. aureus and disrupting the eDNA in biofilms. Similarly, Zhang et al. designed a nanoplatform (Arg-PCN@Gel) targeting S. aureus as a photocatalytic antibacterial system [112]. They demonstrated that Arg-PCN@Gel can target MRSA and can generate ROS to prevent and treat biofilms formed by S. aureus. Recently, Zhuang and co-workers reported that the combination of the natural substrate carvacrol (CA) and a classic Type II PS (such as Ce6) could enhance the generation of ·O2− by promoting the electron transfer process, significantly mitigating the limitations of hypoxic infection environments on Type II photocatalytic efficiency (Fig. 10F–H) [113]. This study creatively converted a Type II PS into a Type I PS by enhancing the electron transfer process, enabling the Type II PS to better tolerate hypoxic microenvironments and offering new perspectives for improving photocatalytic therapy effectiveness in oxygen-deficient conditions.
Fig. 10.
Photocatalytic enhancement strategy based on Type II photocatalytic materials. A) Schematic depiction of BMCV nanoparticle synthesis. B) TEM images and elemental mapping images of BMCV. C) ESR spectra of 1O2 generation by BMCV nanoparticles. D) Evaluation of the targeting ability of BMCV nanoparticles to the infection site. E) Impact of BMCV nanoparticles on S. aureus biofilms and eDNA. F) Schematic illustration of the promotion of ·O2− generation through an electron transfer strategy. G)In vitro antibacterial assay of CA/PS1 complex against S. aureus. H)In vivo antibacterial evaluation of CA/PS1 complex. A-E) Reproduced with permission [111]. Copyright 2024, ACS Publications. F-H) Reproduced with permission [113]. Copyright 2024, Springer Nature.
Photocatalytic therapy is capable of efficiently generating ROS, which disrupt bacterial structures and exhibit significant inhibitory effects against a broad spectrum of bacteria, including antibiotic-resistant strains. Unlike traditional antibiotics, this therapeutic approach operates through mechanisms that do not rely on specific biochemical pathways, thereby reducing the risk of bacterial resistance development. However, the efficacy of photocatalytic therapy is dependent on the availability of specific wavelengths of light, limiting its application under certain conditions. Moreover, the recombination of photogenerated electrons and holes within the material can diminish the efficiency of ROS generation, thus constraining the antibacterial performance of the photocatalyst [114]. Additionally, the potential toxicity of photocatalysts and their long-term safety in biological systems require further investigation and evaluation. Through ongoing optimization of material design and process improvements, this therapy holds promise as a significant tool in future antibacterial treatments.
3.2. Sonocatalytic therapy (SCT)
Ultrasound, a mechanical wave with long propagation distance, strong tissue penetration, and highly concentrated energy, has traditionally been used for disease imaging and diagnosis. Unlike external lasers, ultrasound offers deeper tissue penetration and causes minimal damage to tissues and organs, which has drawn growing attention in the biomedical field in recent years [115,116]. Sonocatalytic therapy (SCT) combines ultrasound with sonosensitizers to achieve therapeutic effects by generating ROS through ultrasound-activated catalysts [[117], [118], [119], [120]]. The core mechanism involves the interaction between ultrasound waves (typically with frequencies ranging from 20 kHz to 1 MHz) and the catalyst, producing ROS, capable of destroying pathogens or cancer cells. Ultrasound can non-invasively penetrate tissues and reach deep-seated lesions, offering excellent therapeutic efficacy for deep-seated diseases, particularly in the treatment of deep solid tumors, which is currently being widely explored [[121], [122], [123], [124], [125]]. Host bacterial infections are not limited to superficial infections such as skin wound infections but also include deep tissue infections like subcutaneous abscesses and infections of bone and muscle tissues (osteomyelitis and implant-related infections), where sonocatalytic therapy holds significant therapeutic advantages and potential. The efficiency of sonocatalytic therapy critically depends on the selection and development of sonosensitizers. An increasing number of sonosensitizers are currently being synthesized and applied in the biomedical field, encompassing organic, inorganic, and organic-inorganic hybrid types. Different types of sonosensitizers exhibit varying sonocatalytic properties and therapeutic effects.
Organic sonosensitizers are types of organic molecules or compounds used in sonocatalytic therapy to enhance the catalytic effect of ultrasound, which primarily include porphyrins and their derivatives, phthalocyanines, 5-aminolevulinic acid, indocyanine green, xanthene dyes, and natural products [[126], [127], [128]]. Organic sonosensitizers have the advantages of high ROS generation efficiency, the ability to undergo structural modification to optimize therapeutic efficacy, and high biocompatibility. Additionally, organic sonosensitizers can be used not only for sonodynamic therapy but also for photodynamic therapy, enabling multimodal treatment. Organic sonosensitizers have the advantages of high ROS generation efficiency, structural modifiability for optimizing therapeutic efficacy, and high biocompatibility [129]. Additionally, organic sonosensitizers can be used not only for sonocatalytic therapy but also for photocatalytic therapy, enabling multimodal treatment [130]. To address the challenges of ineffective accumulation of sonosensitizers at infection sites, hypoxic microenvironments, and rapid oxygen consumption during SDT, Sun et al. proposed a sonication-activated switchable nanozyme system, which can controllably generate O2 and sonosensitizer-mediated ROS during ultrasound activation [131]. By combining Pd@Pt nanoplates with the organic sonosensitizer meso-tetra(4-carboxyphenyl)porphine (T790), they developed a sonocatalytic nano-platform (Pd@Pt-T790). Under ultrasound activation, the catalase-like activity of Pd@Pt is activated, catalyzing the decomposition of H2O2 at the infection site to produce sufficient O2, enhancing the sonocatalytic efficiency, and generating a large amount of toxic ROS. This system demonstrated excellent therapeutic effects on MRSA-induced muscle infections and provided a promising approach for enhancing sonocatalytic therapy for deep bacterial infections. To endow the nano-antibacterial materials with long-term resistance against bacterial infections, Xu and co-workers proposed a synergistic therapeutic strategy combining sonodynamic therapy with adaptive immune modulation (Fig. 11A–C) [132]. They synthesized a responsive, degradable hydrogel containing an organic sonosensitizer (Ce6) on the surface of titanium implants. This multifunctional coating can kill bacteria via sonodynamic therapy and release bacterial antigens, while the manganese ions in the material can promote the maturation of dendritic cells (DCs), further activating both cellular and humoral adaptive immunity. This combined treatment strategy based on sonocatalytic therapy effectively clears diabetes-related IAIs and provides the host with long-term immune memory and antibacterial protection. Similarly, our group reported an in situ vaccination strategy for bacterial infection immunotherapy using an osteomyelitis model. We loaded the organic sonosensitizer PpIX into hollow MnOx nanoparticles and coated them with a hybrid membrane derived from macrophages and tumor cells, naming it HMMP (Fig. 11D–I) [133]. The synthesized HMMP nanoparticles are able to generate ROS via sonocatalysis of PpIX, release bacterial antigens as an in situ vaccine, initiate the antigen presentation process, and activate cellular and humoral adaptive immunity against bacterial infection. This in situ vaccine strategy, based on sonocatalytic therapy, equips the host with long-lasting bacteria-specific immune memory responses, helping to prevent infection recurrence.
Fig. 11.
Sonocatalytic therapy for bacterial infections based on organic sonosensitizers. A) Synthesis and preparation process of Ti–MnO2-CPA@Ce6 substrate. B) The Ti–MnO2-CPA@Ce6 substrate disrupts and kills S. aureus through sonocatalytic therapy and continuously combats infection by activating adaptive immunity through antigen release and manganese ions. C) Schematic illustration of the mechanism by which the Ti–MnO2-CPA@Ce6 substrate activates adaptive immunity. D) Schematic depiction of HMMP nanoparticle synthesis. E) TEM images and elemental mapping images of HMMP nanoparticles. F) TEM images of bacteria after treatment with HMMP nanoparticles. G-H) Representative flow cytometry plots and corresponding quantitative data of CD4 and CD8 T memory cells in the spleen after secondary infection with S. aureus. I) IgG levels in serum and bone marrow after secondary infection with S. aureus. A-C) Reproduced with permission [132]. Copyright 2024, Elsevier Ltd. D-I) Reproduced with permission [133]. Copyright 2022, ACS Publications.
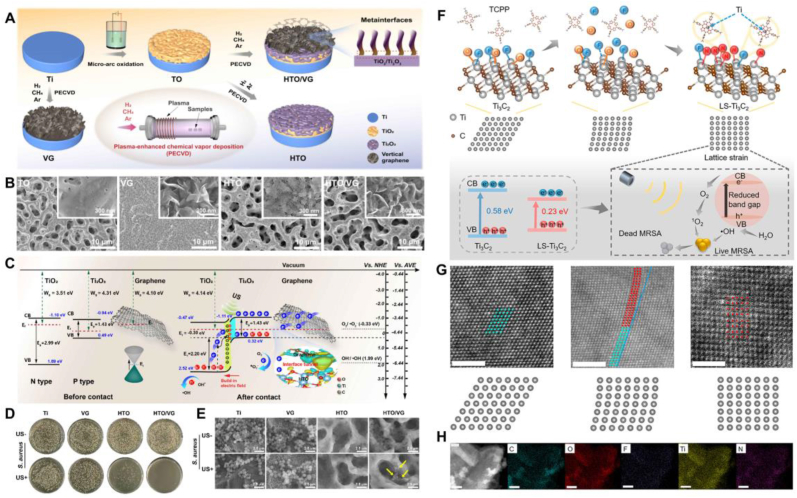
Inorganic sonosensitizers exhibit multiple advantages in sonodynamic therapy, making them highly prominent in the field of antibacterial treatment [134,135]. Inorganic sonosensitizers possess excellent chemical stability, enabling sustained activity in complex biological environments, and they have a high capacity for generating ROS, which significantly enhances their antibacterial efficacy. Moreover, these materials typically exhibit good biocompatibility, ensuring their safe application within biological systems. The continuous development and optimization of inorganic sonosensitizers have significantly broadened the application scope and therapeutic efficacy of sonocatalytic therapy [136]. Currently, inorganic sonosensitizers mainly include noble metal-based sonosensitizers, transition metal-based sonosensitizers, carbon-based nanomaterials, and silicon-based nanomaterials. Through an oxygen vacancy engineering strategy, Qian et al. synthesized TiO2−x microspheres with an abundance of Ti3+ using a simple reductant co-assembly method and deposited graphene quantum dot (GQD) nano-antibiotics on the TiO2−x microspheres to obtain GQD/TiO2−x [137]. This sonocatalytic nano-platform significantly enhances the antibacterial efficiency of SDT against bacteria through accelerated electron-hole pair separation, the POD-like enzyme activity of Ti3+, and the depletion of GSH by Ti4+. Constructing multifunctional sonocatalytic coating nanomaterials on titanium-based implants can be applied to combat IAIs. Zheng et al. integrated a dual-functional coating of two-dimensional (2D) photoacoustic-sensitive black phosphorus nanosheets (BPNSs) and polydopamine (PDA) on the surface of titanium implants with superior biocompatibility [138]. Under ultrasound and laser stimulation, the coating disrupts the cell membrane and antioxidant system of S. aureus through ROS generation and hyperthermia, exhibiting excellent bactericidal efficiency. Guan et al. developed a TiO2/Ti2O3/vertical graphene metainterface heterostructure film on titanium implants, which exhibited remarkable sonodynamic and sonothermal conversion effects under low-intensity ultrasound (Fig. 12A–E) [139]. Antibacterial experiments demonstrated that this metainterface heterostructure enhanced sonocatalytic efficiency, killing over 99.99 % of S. aureus and 99.54 % of E. coli, providing a promising approach for applying sonocatalytic therapy to implant-related biofilm infections. To design more efficient inorganic sonosensitizers, Ku and co-workers synthesized lattice-strain-rich Ti3C2 (LS-Ti3C2) via a one-step solvothermal method using Ti3C2 and Tetrakis (4-carboxyphenyl)porphyrin (TCPP) (Fig. 12F–H) [140]. The introduction of TCPP led to the breakdown of all Ti-O chemical bonds and most Ti-F chemical bonds in the surface layer of Ti₃C₂. Subsequently, the amino groups of TCPP reacted with the exposed Ti atoms, disrupting their arrangement and causing atomic displacement, which ultimately resulted in lattice distortion in Ti₃C₂. This intrinsic lattice strain narrowed the band gap of Ti₃C₂, promoting the separation of electron-hole pairs and facilitating electron transfer under ultrasonic irradiation, thereby enhancing ROS generation efficiency and improving antibacterial activity.
Fig. 12.
Sonocatalytic therapy for bacterial infections based on inorganic sonosensitizers. A) Schematic illustration of the HTO/VG heterostructure preparation process. B) TEM images of HTO/VG heterostructure. C) Schematic depiction of the ROS generation mechanism via sonocatalysis in the HTO/VG heterostructure. D) CFU plate images of bacteria after treatment with HTO/VG heterostructure and US stimulation. E) SEM images of bacteria after treatment with HTO/VG heterostructure and US stimulation. F) Synthesis and preparation process of LS-Ti3C2 and its schematic illustration of the mechanism for killing MRSA based on sonocatalytic therapy. G) HAADF-STEM image of LS-Ti3C2. H) Elemental mapping images of LS-Ti3C2. A-E) Reproduced with permission [139]. Copyright 2024, ACS Publications. F-H) Reproduced with permission [140]. Copyright 2023, ACS Publications.
Organic-inorganic hybrid sonosensitizers combine the benefits of both organic and inorganic materials. These hybrid sonosensitizers are created by integrating organic molecules or polymers with inorganic nanomaterials, resulting in composite materials with unique physicochemical properties. They mainly include MOFs, COFs, and other functional hybrid nanoparticles [141]. MOF nanomaterials, with its high sonosensitizer loading capacity and fast ROS diffusion, have huge potential in sonocatalytic therapy. Meng et al. reported a MIL@Ag heterostructure composed of a titanium-based MOF (MIL) with in situ assembled silver nanoparticles (Ag NPs), featuring high electron-hole pair separation efficiency and enhanced ROS catalytic generation capability [142]. Under ultrasound stimulation, Ag NPs in the MIL@Ag heterostructure can capture activated electrons from MIL, reducing surrounding O2 and producing ·O2−, while the activated holes enable oxidizing H2O to generate ·OH. This MOF-based sonocatalytic enhancement strategy can effectively kill S. aureus, providing a new idea to improving the therapeutic efficacy of sonocatalytic therapy. Zeng et al. synthesized a porphyrin-based MOF with significantly enhanced sonocatalytic capacity through defect homojunctions and found that with increasing amounts of acetic acid, the crystal structure of the MOF transformed from PCN-222 to PCN-224 (Fig. 13A–C) [143]. The higher defect content in PCN-224 improves the separation of sonication-triggered electron-hole pairs, significantly enhancing the efficiency of ROS generation through sonocatalysis. They demonstrated that this defect-induced homojunction MOF exhibited excellent therapeutic efficacy in treating S. aureus-induced osteomyelitis in mice, significantly reducing the bacterial load in the mice. Based on the complex BME to design and fabricate sonosensitizers can more specifically and effectively enhance the sonocatalytic antibacterial efficacy. Xu and co-workers innovatively designed and developed a Ru(II)-based metallacycle sonosensitizer (Ru-A3-TTD) that significantly improves antibacterial efficiency by increasing O2 in the BME, disrupting the antioxidant system within the BME, and enhancing the penetration of the sonosensitizer into biofilms (Fig. 13D–G) [144]. Additionally, Ru-A3-TTD demonstrated excellent biofilm eradication efficacy against MDR E. coli, providing a promising idea for addressing biofilm infections based on sonocatalytic strategies. Efficient sonodynamic therapy can disrupt bacterial structures and promote the release of bacterial-associated double-stranded DNA (dsDNA), thereby activating a robust adaptive immune response. Based on this, Xu et al. designed and fabricated an iron-based COF doped with curcumin and platinum (CFCP) for treating persistent IAIs. They demonstrated that sonocatalytic therapy could kill S. aureus, promote DCs maturation, and enhance neutrophil immune stimulation. Additionally, this treatment strategy can activate humoral immunity by stimulating B cells and T cells, producing antibodies to combat secondary IAIs and providing the host with long-term immune protection.
Fig. 13.
Sonocatalytic therapy for bacterial infections based on organic-inorganic hybrid sonosensitizers. A) Transformation process of the MOF crystal structure with increasing acetic acid concentration and the corresponding defect-enhanced sonocatalytic mechanism. B) SEM and TEM images of various MOF nanomaterials. C) Schematic diagrams of the molecular frameworks of different MOF nanomaterials. D) Schematic illustration of the design of Ru-A3-TTD nano-sonosensitizers and the mechanism for treating biofilm infections. E) LUMO-HOMO distribution based on DFT calculations. F) SEM images of MRSA and MDR E. coli biofilms following treatment with Ru-A3-TTD. G) 3D reconstruction images of MDR E. coli biofilms after Ru-A3-TTD treatment at different time points. A-C) Reproduced with permission [143]. Copyright 2022, Wiley-VCH. D-G) Reproduced with permission [144]. Copyright 2024, Wiley-VCH.
Sonocatalytic therapy, as an emerging antibacterial strategy, offers significant advantages in treating deep-seated infections such as osteomyelitis and implant-associated infections. However, the clinical application of SCT faces challenges, including limitations in ultrasound penetration depth and concerns regarding the biodistribution and potential toxicity of sonosensitizers. Future research should focus on optimizing the design of sonosensitizers to enhance their selectivity and safety, as well as exploring the combination of SCT with other therapeutic modalities to achieve broader clinical applicability and improved therapeutic outcomes.
3.3. Electrocatalytic therapy (ECT)
Electrocatalytic Therapy (ECT) is an emerging treatment method that utilizes electrocatalysis to generate ROS and other active intermediates to treat various diseases. Due to its high efficiency, precision, and minimally invasive nature, ECT has shown great potential in Biomedical field. The mechanism of action involves using electrocatalysts under an applied electric field to perform redox reactions, generating highly active ROS that can disrupt cellular components such as cell membranes, DNA, and proteins, thereby killing bacteria or cancer cells. Unlike chemodynamic therapy, which is affected by the microenvironment of infection, and photocatalytic or sonocatalytic therapies, which can cause harm to human cells due to high power density of light or US, ECT possesses advantages of being non-invasive, having high tissue penetration, and high selectivity. ECT was first proposed by Liu and colleagues, also known as electrodynamic therapy (EDT), and was applied in cancer treatment [145]. Subsequently, cancer treatment strategies based on ECT have gradually emerged [146,147]. Similarly, ECT has evident advantages in combating bacterial infections and clinical translation. The most commonly used nano-electrosensitizers in ECT are noble metal Pt nanoparticles [146]. Based on this, Qiao et al. developed a novel biomimetic nano-platform by using ginger-derived extracellular vesicles (EVs) to encapsulate Pd-Pt nanosheets (Fig. 14A–B) [148]. Experimental data and computational simulations demonstrated that Pd-Pt nanosheets exhibit higher electrocatalytic activity compared to Pt nanoparticles. Additionally, the encapsulation with cell membrane vesicles ensures the accumulation of the nano-platform at the infection site, significantly improving the treatment of S. aureus-induced subcutaneous abscesses in mice. To further enhance the efficiency of electrocatalytic antibacterial therapy, Duan and co-workers proposed a strategy to reverse the spin state of Ni−Co3O4, which can improve the production efficiency of 1O2 through asymmetric orbital hybridization, thereby exhibiting strong bactericidal effects (Fig. 14C–F) [149]. The mechanism involves the transition of Co³⁺ from a low-spin to a high-spin state by introducing Ni, which enhances Co 3d-O 2p orbital hybridization between cobalt ions and adsorbed oxygen, thus increasing the oxygen catalytic activity of Ni−Co₃O₄. This study highlights the significant potential and value of spin engineering strategies in optimizing the efficiency of 1O₂ production by electrocatalysts and addressing microbial infections. Zhou and co-workers combined electrocatalytic therapy and photocatalytic therapy to develop a novel anti-sandwich structure of SeV2+-PT loaded photo-electronic wound dressing (SeV2+-PT PEWD) [150]. This dressing, under dual stimulation of visible light and electricity, efficiently generates ROS, enabling rapid bacterial eradication and reducing the wound healing time of bacterial infections to seven days.
Fig. 14.
Antibacterial strategy based on electrocatalytic therapy. A) Schematic depiction of EV-Pd-Pt nanoparticle synthesis by attaching carboxyl-functionalized Pd-Pt nanoparticles to amino group-rich ginger-derived EVs. B)In vivo synergistic ECT/PTT strategy to eliminate infection. C) Schematic depiction of the bactericidal mechanism of the Ni−Co3O4 system. D) Schematic representation of the antibacterial mechanism of the Ni−Co3O4 system. E) EPR spectra of Ni−Co3O4 producing 1O2 and ⋅OOH/O2−. F) Free energy diagram of molecular oxygen activation processes for Ni−Co3O4 and Co3O4. A-B) Reproduced with permission [148]. Copyright 2022, Springer Nature. C-F) Reproduced with permission [149]. Copyright 2024, Wiley-VCH.
Piezoelectric catalysis refers to the phenomenon where piezoelectric materials with non-centrosymmetric structures generate charges under external mechanical stress activation [151]. The generated charges further react with surrounding water or oxygen molecules to produce ROS. Therefore, piezoelectric catalytic materials can also be used to treat cancer and microbial infections [[152], [153], [154]]. Ultrasound generates mechanical strain that polarizes piezoelectric materials, creating an internal electric field that effectively separates electron-hole pairs. The resulting piezoelectric potential drives redox reactions. When the conduction band (CB) edge is more negative than the O₂/·O₂⁻ redox potential (−0.33 V), ·O₂⁻ is produced. Similarly, when holes transfer to H₂O and the valence band (VB) edge is more positive than the H₂O/·OH redox potential (2.01 V), ·OH is generated. This mechanism is distinct from that of sonocatalytic therapy. Among various piezoelectric materials, functionalized barium titanate nanoparticles (BTO, BaTiO3) have become classic piezoelectric catalytic materials due to its high piezoelectric performance, environmental friendliness, and simple structure. Lei et al. reported a novel piezoelectric catalytic nanomaterial consisting of barium titanate nanoparticles and selenium nanoparticles, which enhances electron-hole separation and promotes internal free carrier transfer. This results in stronger piezoelectric catalytic performance, effectively combating bacterial infections [155]. Similarly, Chen et al. also modified and optimized barium titanate (BaTiO3) by depositing Ag NPs on the surface of BaTiO3 to form a heterostructure (Fig. 15A) [156]. This configuration enabled the piezoelectric material to generate a strong piezoelectric catalytic reaction under low concentrations and short ultrasound stimulation. Additionally, they incorporated the synthesized high-efficiency piezoelectric nanoparticles into biodegradable silk fibroin (SF), resulting in a piezoelectric catalytic composite hydrogel. This hydrogel exhibited excellent piezoelectric catalytic performance, degradability, biocompatibility, and remarkable antibacterial activity, significantly accelerating the healing of infected wounds. The basic principle of thermoelectrocatalysis involves utilizing the potential difference generated by thermoelectric materials under a temperature gradient to drive electrocatalytic reactions. This technology combines the advantages of thermoelectric effects and electrocatalytic reactions, enabling efficient utilization of thermal energy while achieving high-selectivity chemical transformations. Bismuth telluride (Bi2Te3) is one of the most common and excellent thermoelectric materials near room temperature. Wang and co-workers developed an efficient thermoelectrocatalytic material (rGO-Bi2Te3) by in situ growing Bi2Te3 nanosheets on reduced graphene oxide (rGO). In photothermal-driven thermoelectrocatalysis, the rGO-Bi2Te3 nanosheets can accelerate the separation and transfer of photogenerated charges, further promoting the effective generation of ROS, resulting in excellent therapeutic effects on MRSA-induced infected wounds (Fig. 15B–F) [157].
Fig. 15.
Antibacterial strategy based on piezocatalytic therapy. A) Synthesis process of Ag@BT NPs and schematic illustration of the application and antibacterial mechanism of SF-MA/PEGDA hydrogels for treating infected wounds. B) Schematic illustration of the heterointerface scattering and photothermal electrocatalytic mechanism. C) Synthesis process of rGO-Bi2Te3 nanosheets. D) TEM images and EDS elemental mapping images of rGO-Bi2Te3 nanosheets. E-F) Plate images and SEM images of S. aureus after treated with different materials. A) Reproduced with permission [156]. Copyright 2024, Elsevier Ltd. B-F) Reproduced with permission [157]. Copyright 2022, Wiley-VCH.
Electrocatalytic Therapy, as an emerging antibacterial strategy, holds significant potential for further development and research in the antibacterial field. It should be noted that the stability of piezoelectric materials' performance and their mechanical energy conversion efficiency in various biological environments still require further validation. Moreover, the biocompatibility and potential toxicity of piezoelectric materials need to be thoroughly investigated, particularly concerning the cumulative effects that may arise from long-term in vivo applications. In the future, ECT is expected to play a crucial role in combating antibiotic-resistant bacteria and complex infections, positioning itself as a valuable tool in the field of antibacterial treatment.
4. Catalysis-assisted immunotherapy (CAIT)
During the infection process, the interaction between pathogens and the host immune system creates a complex infection microenvironment, which not only directly affects the growth and spread of pathogens but also has profound impacts on the host immune response. In some cases, the infection microenvironment can lead to immunosuppression, rendering the host immune system unable to effectively clear the pathogens. This phenomenon of immunosuppression is particularly evident in chronic and refractory infections. Specifically, in biofilm-associated infections, biofilms induce hypoxia, nutrient deficiency, and an acidic microenvironment, polarizing host immune cells towards an anti-inflammatory phenotype, thus reducing the ability of macrophages and other cells to clear bacteria and secrete pro-inflammatory factors [158]. Moreover, the adaptive immune system also plays a crucial role in combating infections, particularly by generating specific memory cells and antibodies, which are vital for preventing recurrent infections. Catalysis-assisted immunotherapy (CAIT) is a novel therapeutic strategy that combines catalytic reactions and immune modulation, aiming to activate and enhance the immune response through the action of catalysts to achieve disease treatment. This strategy leverages the ROS generated by catalysts, which can not only directly kill pathogens or cancer cells but also modulate the immune system to enhance the disease resistance of the host. In cancer treatment, catalytic therapy is often combined with immunotherapy, which on one hand can enhance anti-tumor efficacy and inhibit tumor recurrence and metastasis, and on the other hand, reduce side effects on the host [[159], [160], [161]]. Catalytic-assisted immunotherapy also holds significant potential and value in the field of infections and has started to attract increasing interest in recent years.
Innate immunity is the first line of defense in the host immune system, rapidly recognizing and responding to pathogens. Among the key players in this process are macrophages and neutrophils, which play crucial roles in antibacterial action. In the biofilm infection microenvironment, macrophage activity is often suppressed, leading to compromised macrophages. Based on this, Mei et al. proposed an intelligent Cu-doped Mo (VI)-based polyoxometalate nanocluster (Cu-POM) (Fig. 16A–D) [162]. Cu-POM can catalytically generate ROS within the biofilm microenvironment, possessing direct antibacterial properties while reactivating compromised macrophages to enhance their chemotactic ability, phagocytic activity, and pro-inflammatory cytokine release capability, effectively clearing planktonic S. aureus. Additionally, their group reported a novel “interference-regulation strategy” using bovine serum albumin-iridium oxide nanoparticles (BIONPs) as biofilm homeostasis disruptors and immune modulators [163]. The synthesized BIONPs exhibit catalase-like activity, catalyzing H2O2 to produce O2, thereby enhancing the efficacy of PDT by generating abundant 1O2 to effectively eradicate biofilms. More importantly, BIONPs successfully repolarized macrophages in vivo to the pro-inflammatory M1 phenotype, facilitating the phagocytosis of residual biofilm and preventing biofilm reformation. In the diabetic-related biofilm infection microenvironment (DRBI), neutrophils are prone to shift from phagocytosis to NETosis under high ROS stimulation, producing abundant net-like structures (NETs) and forming “NETs barriers” on the surface and inside the biofilm, thereby enhancing the EPS barrier around biofilms [[164], [165], [166]]. The reinforced biofilm structure and inhibited neutrophil phagocytosis perpetuate biofilm infections in DRBIs, leading to persistent and chronic infections. To address the DRBIs microenvironment, Guo and co-workers proposed a neutrophil immune function conversion (NIFC) strategy by designing DNase-I-loaded V2C MXene (DNase-I@V2C) to combat biofilm infections in diabetes (Fig. 16E–F) [167]. DNase-I-V2C, through SOD and CAT-like enzymatic activities, scavenges ROS, reverses neutrophil function, disrupts the biofilm “EPS and NETs barriers” and enhances neutrophil clearance of bacteria and biofilm debris.
Fig. 16.
Catalysis-assisted antibacterial strategies for activating innate immunity. A) Synthesis process of BME-responsive self-assembled nano-reactors and the mechanism of promoting macrophage reactivation in the biofilm infection microenvironment to eliminate IAIs. B-C) Flow cytometry images of intracellular ROS and surface markers CD80 and CD206 in macrophages after Cu-POM nanocatalytic platform intervention. D) Fluorescence images of phagocytosis of S. aureus by reactivated macrophages after Cu-POM nanocatalytic platform reactivation. E) Design and synthesis process of DNase-I@V2C. F) Mechanism diagram of DNase-I@V2C nanoplatform for treating diabetes-related IAIs through neutrophil function conversion. A-D) Reproduced with permission [162]. Copyright 2023, Wiley-VCH. E-F) Reproduced with permission [167]. Copyright 2023, Wiley-VCH.
Despite the significant progress in catalytic therapy for anti-infective immune modulation in recent years, strategies primarily focusing on innate immunity still lack the ability to provide long-term immunity, failing to effectively prevent infection recurrence. Therefore, future directions in catalytic-assisted immunotherapy may lie in simultaneously enhancing innate immune responses and activating adaptive immunity to achieve effective infection clearance and induce long-term immune memory. Based on this, Xu et al. proposed an iron-based covalent organic framework nanoadjuvant doped with curcumin and platinum (CFCP) to achieve effective treatment of IRIs by inducing a systemic immune response (Fig. 17A–D) [168]. The ROS generated through sonocatalysis can disrupt bacterial structures, induce the release of bacteria-related double-stranded DNA (dsDNA), and activate adaptive immunity through the cGAS-STING pathway to prevent infection recurrence. Similarly, our group have synthesized PVP-modified two-dimensional manganese phosphotelluride (MnPSe3) nanosheets (MPS-PVP), which can trigger strong antibacterial humoral immunity in a mouse model of implant infection by modulating antigen presentation and co-stimulatory molecule expression in the BME (Fig. 17F–H) [169]. We demonstrated that using preoperative neoadjuvant immunotherapy with MPS-PVP successfully reduced the severity of primary and secondary infections in mice with implant infections, providing an antibiotic-free alternative for the treatment of IAIs.
Fig. 17.
Catalysis-related antibacterial strategies for activating humoral and adaptive immunity. A) Schematic illustration of the synthesis of CFCP nanoparticles. B) CFCP nanoparticles promote the release of S. aureus antigens through sonocatalytic therapy and activate mouse adaptive immunity via the cGAS-STING pathway, treating IAIs and preventing infection recurrence. C-D) Graphs showing the area of infection regions on the back and CFU count results in different groups of mice after recurrent infection following CFCP nanoparticle treatment. E) Levels of IgM and IgG antibodies in different groups of mice after CFCP nanoparticle treatment. F) Immune-stimulating MPS-PVP nanosheets induce "in situ" amplification of bacteria-specific antibodies in biofilms by generating ROS, activating immune memory in mice, and thereby eliminating IAIs. G-H) Flow cytometry plots and corresponding data showing the activation of adaptive immunity in IAIs mice after treatment with MPS-PVP nanosheets. A-E) Reproduced with permission [168]. Copyright 2024, Elsevier Ltd. F-H) Reproduced with permission [169]. Copyright 2022, Springer Nature.
Catalysis-assisted immunotherapy enhances the antibacterial effect by activating host anti-infection immunity, which has gradually become one of the research hotspots. However, this therapeutic approach still faces several challenges that require further investigation and resolution in the future, particularly in adjusting the host immune homeostasis to avoid excessive inflammatory responses. Additionally, more research is needed to focus on activating adaptive immunity and strengthening the host immune protection. In the future, CAIT will be a significant direction in the field of nanocatalytic antibacterial therapy, with promising potential for clinical translation.
5. Conclusion and perspective
Bacterial infections have posed a long-standing challenge for clinicians, especially in the post-antibiotic era with the prevalence of drug-resistant bacteria. Research on nano-biomaterials for antibacterial applications has been ongoing for many years, among which metal ions (such as Ag⁺, Cu2⁺, and Zn2⁺) and their corresponding metal-based nano-biomaterials have made significant advances in recent years. Due to their unique bioactivity and diverse antibacterial mechanisms, metal ions have been widely applied in antibacterial treatments, medical device coatings, and packaging materials, helping to mitigate the issue of antibiotic-resistant infections caused by the overuse of antibiotics. However, the potential biotoxicity, metabolism, and accumulation of metal ions in the human body remain major challenges that limit their broader application. Photothermal antibacterial therapy is another area of extensive research in recent years, focusing on the use of nanomaterials with photothermal conversion capabilities. Upon exposure to light at specific wavelengths, these materials absorb light energy and convert it into heat, leading to a localized temperature increase that directly damages bacterial cell membranes, proteins, and nucleic acids, ultimately causing bacterial death. Despite its numerous advantages, photothermal therapy also has notable drawbacks, including damage to surrounding healthy tissues and the limited penetration depth of near-infrared (NIR) light. Therefore, the development of multifunctional photothermal antibacterial materials based on mild photothermal therapy and NIR-II region light-excited photothermal materials is a promising direction for the future of photothermal antibacterial therapy.
Nanocatalytic medicine demonstrates distinct advantages over traditional nanomaterial-based treatments for bacterial infections, including lower dosage requirements, higher bactericidal efficiency, and selective catalytic effects in the infection microenvironment. Despite significant progress in recent years, the further development and clinical translation of nanocatalytic medicine still face numerous challenges. Due to the limited understanding of the complex physiological and pathophysiological mechanisms within the infection microenvironment, the catalytic activity of currently reported nanocatalytic biomaterials remains below clinical expectations. Furthermore, the potential biotoxicity and metabolic accumulation of nanocatalytic drugs in the human body are critical issues that must be addressed for future clinical application. The core mechanism of nanocatalytic medicine lies in the production of ROS; however, excessive ROS generation can lead to inflammatory storms and pathological damage to the host. Balancing the efficiency of ROS production and its bactericidal effect is a crucial challenge worthy of attention.
The future direction of nanocatalytic medicine should focus on designing more efficient, intelligent, precise, and safe nanocatalytic materials. It is essential to improve the catalytic efficiency of nanocatalysts and enhance their antibacterial efficacy, particularly for biofilm-associated infections, where greater attention should be paid to biofilm infiltration and structural disruption. The combination of various catalytic therapies represents a key trend, whether exogenous catalysis (such as CDT and NCT) or endogenous catalysis (such as PCT, SCT, ECT), alongside immunotherapy and photothermal therapy. The synergistic treatment with multimodal nanobiomaterials can greatly improve material performance and reduce the occurrence of bacterial resistance.
Intelligence is a critical development direction for nanocatalytic medicine, aiming to precisely control and regulate catalytic performance, thereby improving therapeutic selectivity and efficacy while minimizing side effects. Notably, the treatment of bacterial infections requires a multifaceted approach, including bacterial clearance during acute and subacute phases, as well as tissue repair during chronic stages. Smart nanocatalytic materials should respond to multiple stimuli within the complex infection microenvironment, adjusting their activity according to changes in the pathological environment. Precision is essential, requiring the nanocatalysts to achieve targeted localization within the body to minimize impact on healthy tissues. This can be achieved by modifying the surface of nanomaterials with specific ligands, such as antibodies, peptides, or small molecules, that selectively bind to receptors in diseased tissues. Another promising approach is using external magnetic fields to guide magnetic nanoparticles to the infection site for efficient targeted delivery and catalytic reactions. Concerns about biosafety are widespread in nanomaterials, as they may be phagocytosed by different types of cells, leading to excessive ROS production or exacerbated inflammation, which could cause cytotoxicity and hinder the recovery of infected tissues. Reducing the toxicity of catalytic materials and minimizing their long-term accumulation within the host are critical considerations in future material design. Furthermore, most current biosafety studies of catalytic nanodrugs are conducted in cultured cells or mouse models, highlighting the need to establish biosafety testing systems that more closely mimic the human physiological environment.
In recent years, the rapid development of AI technology has significantly accelerated the design, optimization, and application of materials, increasing research efficiency and reducing costs. The combination of theoretical simulations and machine learning could expedite the structural optimization of nanocatalysts and the prediction of their catalytic activity. The future of catalytic nanomedicine will integrate chemistry, materials science, computer science, and medicine. This convergence not only advances material science but also provides new approaches to addressing major challenges such as global antimicrobial resistance.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent for publication
Not applicable.
CRediT authorship contribution statement
Min Ge: Writing – review & editing, Writing – original draft, Conceptualization. Feng Jiang: Writing – original draft, Conceptualization. Han Lin: Writing – review & editing, Conceptualization.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgements
We greatly acknowledge the financial support from Shanghai Pilot Program for Basic Research-Chinese Academy of Science, Shanghai Branch (Grant No. JCYJ-SHFY-2022-003), National Natural Science Foundation of China (Grant No. 52372276, 82272511), Youth Innovation Promotion Association of the Chinese Academy of Sciences (Grant No. 2023262), Young Elite Scientists Sponsorship Program by CAST (Grant No. YESS20210149), Shanghai Science and Technology Committee Rising-Star Program (Grant No. 22QA1410200), and Natural Science Foundation of Shanghai (Grant No. 23ZR1472300).
Contributor Information
Feng Jiang, Email: jiangfeng95428@163.com.
Han Lin, Email: linhan@mail.sic.ac.cn.
Data availability
No data was used for the research described in the article.
References
- 1.Blair J.M., Webber M.A., Baylay A.J., Ogbolu D.O., Piddock L.J. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015;13(1):42–51. doi: 10.1038/nrmicro3380. [DOI] [PubMed] [Google Scholar]
- 2.Li T., Wang Z., Guo J., de la Fuente-Nunez C., Wang J., Han B., Tao H., Liu J., Wang X. Bacterial resistance to antibacterial agents: mechanisms, control strategies, and implications for global health. Sci. Total Environ. 2023;860 doi: 10.1016/j.scitotenv.2022.160461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Global mortality associated with 33 bacterial pathogens in 2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2022;400(10369):2221–2248. doi: 10.1016/S0140-6736(22)02185-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399(10325):629–655. doi: 10.1016/S0140-6736(21)02724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tacconelli E., Carrara E., Savoldi A., Harbarth S., Mendelson M., Monnet D.L., Pulcini C., Kahlmeter G., Kluytmans J., Carmeli Y., Ouellette M., Outterson K., Patel J., Cavaleri M., Cox E.M., Houchens C.R., Grayson M.L., Hansen P., Singh N., Theuretzbacher U., Magrini N. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018;18(3):318–327. doi: 10.1016/S1473-3099(17)30753-3. [DOI] [PubMed] [Google Scholar]
- 6.Almohaya A., Fersovich J., Weyant R.B., Fernández García O.A., Campbell S.M., Doucette K., Lotfi T., Abraldes J.G., Cervera C., Kabbani D. The impact of colonization by multidrug resistant bacteria on graft survival, risk of infection, and mortality in recipients of solid organ transplant: systematic review and meta-analysis. Clin Microbiol Infect. 2024;30(10):1228–1243. doi: 10.1016/j.cmi.2024.03.036. [DOI] [PubMed] [Google Scholar]
- 7.Assis L.M., Nedeljković M., Dessen A. New strategies for targeting and treatment of multi-drug resistant Staphylococcus aureus. Drug Resist. Updates. 2017;31:1–14. doi: 10.1016/j.drup.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Kanj S.S., Bassetti M., Kiratisin P., Rodrigues C., Villegas M.V., Yu Y., van Duin D. Clinical data from studies involving novel antibiotics to treat multidrug-resistant Gram-negative bacterial infections. Int. J. Antimicrob. Agents. 2022;60(3) doi: 10.1016/j.ijantimicag.2022.106633. [DOI] [PubMed] [Google Scholar]
- 9.Bhattacharya M., Horswill A.R. The role of human extracellular matrix proteins in defining Staphylococcus aureus biofilm infections. FEMS Microbiol. Rev. 2024;48(1) doi: 10.1093/femsre/fuae002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang X., Wang D., Lu H., Wang X., Wang X., Su J., Xia G. Strategies to promote the journey of nanoparticles against biofilm-associated infections. Small. 2024;20(10) doi: 10.1002/smll.202305988. [DOI] [PubMed] [Google Scholar]
- 11.Schilcher K., Horswill A.R. Staphylococcal biofilm development: structure, regulation, and treatment strategies. Microbiol. Mol. Biol. Rev. 2020;84(3) doi: 10.1128/MMBR.00026-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lv X., Wang L., Mei A., Xu Y., Ruan X., Wang W., Shao J., Yang D., Dong X. Recent nanotechnologies to overcome the bacterial biofilm matrix barriers. Small. 2023;19(6) doi: 10.1002/smll.202206220. [DOI] [PubMed] [Google Scholar]
- 13.Xu Q., Hua Y., Zhang Y., Lv M., Wang H., Pi Y., Xie J., Wang C., Yong Y. A biofilm microenvironment-activated single-atom iron nanozyme with NIR-controllable nanocatalytic activities for synergetic bacteria-infected wound therapy. Adv Healthc Mater. 2021;10(22) doi: 10.1002/adhm.202101374. [DOI] [PubMed] [Google Scholar]
- 14.Geng C., He S., Yu S., Johnson H.M., Shi H., Chen Y., Chan Y.K., He W., Qin M., Li X., Deng Y. Achieving clearance of drug-resistant bacterial infection and rapid cutaneous wound regeneration using an ROS-balancing-engineered heterojunction. Adv Mater. 2024;36(16) doi: 10.1002/adma.202310599. [DOI] [PubMed] [Google Scholar]
- 15.Yang J., Zhang R., Zhao H., Qi H., Li J., Li J.F., Zhou X., Wang A., Fan K., Yan X., Zhang T. Bioinspired copper single-atom nanozyme as a superoxide dismutase-like antioxidant for sepsis treatment. Exploration (Beijing) 2022;2(4) doi: 10.1002/EXP.20210267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mo F., Lin C., Lu J., Sun D. Integrating artificial DNAzymes with natural enzymes on 2D MOF hybrid nanozymes for enhanced treatment of bacteria-infected wounds. Small. 2024;20(21) doi: 10.1002/smll.202307256. [DOI] [PubMed] [Google Scholar]
- 17.Ezraty B., Gennaris A., Barras F., Collet J.F. Oxidative stress, protein damage and repair in bacteria. Nat. Rev. Microbiol. 2017;15(7):385–396. doi: 10.1038/nrmicro.2017.26. [DOI] [PubMed] [Google Scholar]
- 18.Geng Z., Cao Z., Liu J. Recent advances in targeted antibacterial therapy basing on nanomaterials. Exploration (Beijing) 2023;3(1) doi: 10.1002/EXP.20210117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee J., Liao H., Wang Q., Han J., Han J.H., Shin H.E., Ge M., Park W., Li F. Exploration of nanozymes in viral diagnosis and therapy. Exploration (Beijing) 2022;2(1) doi: 10.1002/EXP.20210086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X., Wu X.T., Xu Q., Zhu Q.L. Hierarchically ordered pore engineering of metal-organic framework-based materials for electrocatalysis. Adv Mater. 2024 doi: 10.1002/adma.202401926. [DOI] [PubMed] [Google Scholar]
- 21.Li Y.H., Zhao S.N., Zang S.Q. Programmable kernel structures of atomically precise metal nanoclusters for tailoring catalytic properties. Exploration (Beijing) 2023;3(3) doi: 10.1002/EXP.20220005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu Y., Liu J., Lee C., Li M., Han B., Wu T., Pan H., Geng D., Yan Q. Integration of metal-organic frameworks and metals: synergy for electrocatalysis. Small. 2023;19(32) doi: 10.1002/smll.202300916. [DOI] [PubMed] [Google Scholar]
- 23.Zhu X., Xu J., Ling G., Zhang P. Tunable metal-organic frameworks assist in catalyzing DNAzymes with amplification platforms for biomedical applications. Chem. Soc. Rev. 2023;52(21):7549–7578. doi: 10.1039/d3cs00386h. [DOI] [PubMed] [Google Scholar]
- 24.Li C., Hang T., Jin Y. Atomically Fe-anchored MOF-on-MOF nanozyme with differential signal amplification for ultrasensitive cathodic electrochemiluminescence immunoassay. Exploration (Beijing) 2023;3(4) doi: 10.1002/EXP.20220151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huo J., Jia Q., Huang H., Zhang J., Li P., Dong X., Huang W. Emerging photothermal-derived multimodal synergistic therapy in combating bacterial infections. Chem. Soc. Rev. 2021;50(15):8762–8789. doi: 10.1039/d1cs00074h. [DOI] [PubMed] [Google Scholar]
- 26.Cheng S., Qi M., Li W., Sun W., Li M., Lin J., Bai X., Sun Y., Dong B., Wang L. Dual-responsive nanocomposites for synergistic antibacterial therapies facilitating bacteria-infected wound healing. Adv Healthc Mater. 2023;12(6) doi: 10.1002/adhm.202202652. [DOI] [PubMed] [Google Scholar]
- 27.Zhang C., Bu W., Ni D., Zhang S., Li Q., Yao Z., Zhang J., Yao H., Wang Z., Shi J. Synthesis of iron nanometallic glasses and their application in cancer therapy by a localized Fenton reaction. Angew Chem. Int. Ed. Engl. 2016;55(6):2101–2106. doi: 10.1002/anie.201510031. [DOI] [PubMed] [Google Scholar]
- 28.Estrella V., Chen T., Lloyd M., Wojtkowiak J., Cornnell H.H., Ibrahim-Hashim A., Bailey K., Balagurunathan Y., Rothberg J.M., Sloane B.F., Johnson J., Gatenby R.A., Gillies R.J. Acidity generated by the tumor microenvironment drives local invasion. Cancer Res. 2013;73(5):1524–1535. doi: 10.1158/0008-5472.CAN-12-2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Webb B.A., Chimenti M., Jacobson M.P., Barber D.L., Dysregulated pH. A perfect storm for cancer progression. Nat. Rev. Cancer. 2011;11(9):671–677. doi: 10.1038/nrc3110. [DOI] [PubMed] [Google Scholar]
- 30.Sun D., Sun X., Zhang X., Wu J., Shi X., Sun J., Luo C., He Z., Zhang S. Emerging chemodynamic nanotherapeutics for cancer treatment. Adv Healthc Mater. 2024 doi: 10.1002/adhm.202400809. [DOI] [PubMed] [Google Scholar]
- 31.Hao J.N., Ge K., Chen G., Dai B., Li Y. Strategies to engineer various nanocarrier-based hybrid catalysts for enhanced chemodynamic cancer therapy. Chem. Soc. Rev. 2023;52(22):7707–7736. doi: 10.1039/d3cs00356f. [DOI] [PubMed] [Google Scholar]
- 32.Luo Y., Ruan Z.S., Guo Z., Chen Y.F., Lin H., Ge M., Zhu C. Electron orbital hybridization-enhanced copper-nanocatalysis for anti-infection. Adv. Funct. Mater. 2024 [Google Scholar]
- 33.Li Y., Xiu W., Yang K., Wen Q., Yuwen L., Luo Z., Liu X., Yang D., Xie X., Wang L. A multifunctional Fenton nanoagent for microenvironment-selective anti-biofilm and anti-inflammatory therapy. Mater. Horiz. 2021;8(4):1264–1271. doi: 10.1039/d0mh01921f. [DOI] [PubMed] [Google Scholar]
- 34.Guo G., Zhang H., Shen H., Zhu C., He R., Tang J., Wang Y., Jiang X., Wang J., Bu W., Zhang X. Space-selective chemodynamic therapy of CuFe(5)O(8) nanocubes for implant-related infections. ACS Nano. 2020;14(10):13391–13405. doi: 10.1021/acsnano.0c05255. [DOI] [PubMed] [Google Scholar]
- 35.Li B., Shu R., Dai W., Yang F., Xu H., Shi X., Li Y., Bai D., Yang W., Deng Y. Bioheterojunction-engineered polyetheretherketone implants with diabetic infectious micromilieu twin-engine powered disinfection for boosted osteogenicity. Small. 2022;18(45) doi: 10.1002/smll.202203619. [DOI] [PubMed] [Google Scholar]
- 36.Sun M., Wang L., Zhuo Y., Xu S., Liu H., Jiang X., Lu Z., Wang X., Wang Y., Yue G., Feng B., Rao H., Wu D. Multi-enzyme activity of MIL-101 (Fe)-Derived cascade nano-enzymes for antitumor and antimicrobial therapy. Small. 2024;20(17) doi: 10.1002/smll.202309593. [DOI] [PubMed] [Google Scholar]
- 37.Zhang D., Xu R., Chen S., Du H., Qian S., Peng F., Liu X. Surface defect engineered-Mg-based implants enable the dual functions of superhydrophobic and synergetic photothermal/chemodynamic therapy. Bioact. Mater. 2023;30:15–28. doi: 10.1016/j.bioactmat.2023.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He X., Lv Y., Lin Y., Yu H., Zhang Y., Tong Y., Zhang C. Platinum nanoparticles regulated V(2) C MXene nanoplatforms with NIR-II enhanced nanozyme effect for photothermal and chemodynamic anti-infective therapy. Adv Mater. 2024 doi: 10.1002/adma.202400366. [DOI] [PubMed] [Google Scholar]
- 39.Deng Y., Ouyang X., Sun J., Shi X., Li Y., Chan Y.K., Yang W., Peng S. Rapid sterilisation and diabetic cutaneous regeneration using cascade bio-heterojunctions through glucose oxidase-primed therapy. Bioact. Mater. 2023;25:748–765. doi: 10.1016/j.bioactmat.2022.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Y., Chen W., Mu W., Zhou Q., Liu J., Li B., Liu T., Yu T., Hu N., Chen X. Physiological microenvironment dependent self-cross-linking of multifunctional nanohybrid for prolonged antibacterial therapy via synergistic chemodynamic-photothermal-biological processes. Nano Lett. 2024;24(23):6906–6915. doi: 10.1021/acs.nanolett.4c00671. [DOI] [PubMed] [Google Scholar]
- 41.Huang Y., Wan X., Su Q., Zhao C., Cao J., Yue Y., Li S., Chen X., Yin J., Deng Y., Zhang X., Wu T., Zhou Z., Wang D. Ultrasound-activated piezo-hot carriers trigger tandem catalysis coordinating cuproptosis-like bacterial death against implant infections. Nat. Commun. 2024;15(1):1643. doi: 10.1038/s41467-024-45619-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.He X., Qian Y., Wu C., Feng J., Sun X., Zheng Q., Li X., Shen J. Entropy-mediated high-entropy MXenes nanotherapeutics: NIR-II-Enhanced intrinsic oxidase mimic activity to combat methicillin-resistant Staphylococcus aureus infection. Adv Mater. 2023;35(26) doi: 10.1002/adma.202211432. [DOI] [PubMed] [Google Scholar]
- 43.Manea F., Houillon F.B., Pasquato L., Scrimin P. Nanozymes: gold-nanoparticle-based transphosphorylation catalysts. Angew Chem. Int. Ed. Engl. 2004;43(45):6165–6169. doi: 10.1002/anie.200460649. [DOI] [PubMed] [Google Scholar]
- 44.Wu X., Li Y., Wen M., Xie Y., Zeng K., Liu Y.N., Chen W., Zhao Y. Nanocatalysts for modulating antitumor immunity: fabrication, mechanisms and applications. Chem. Soc. Rev. 2024;53(5):2643–2692. doi: 10.1039/d3cs00673e. [DOI] [PubMed] [Google Scholar]
- 45.Jiang D., Ni D., Rosenkrans Z.T., Huang P., Yan X., Cai W. Nanozyme: new horizons for responsive biomedical applications. Chem. Soc. Rev. 2019;48(14):3683–3704. doi: 10.1039/c8cs00718g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiao L., Yan H., Wu Y., Gu W., Zhu C., Du D., Lin Y. When nanozymes meet single-atom catalysis. Angew Chem. Int. Ed. Engl. 2020;59(7):2565–2576. doi: 10.1002/anie.201905645. [DOI] [PubMed] [Google Scholar]
- 47.Huang Y., Ren J., Qu X. Nanozymes: classification, catalytic mechanisms, activity regulation, and applications. Chem. Rev. 2019;119(6):4357–4412. doi: 10.1021/acs.chemrev.8b00672. [DOI] [PubMed] [Google Scholar]
- 48.Dong C., Dai X., Wang X., Lu Q., Chen L., Song X., Ding L., Huang H., Feng W., Chen Y., Chang M. A calcium fluoride nanozyme for ultrasound-amplified and Ca(2+) -Overload-Enhanced catalytic tumor nanotherapy. Adv Mater. 2022;34(43) doi: 10.1002/adma.202205680. [DOI] [PubMed] [Google Scholar]
- 49.Chen D., Xia Z., Guo Z., Gou W., Zhao J., Zhou X., Tan X., Li W., Zhao S., Tian Z., Qu Y. Bioinspired porous three-coordinated single-atom Fe nanozyme with oxidase-like activity for tumor visual identification via glutathione. Nat. Commun. 2023;14(1):7127. doi: 10.1038/s41467-023-42889-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang L., Zhang X., You Z., Yang Z., Guo M., Guo J., Liu H., Zhang X., Wang Z., Wang A., Lv Y., Zhang J., Yu X., Liu J., Chen C. A molybdenum disulfide nanozyme with charge-enhanced activity for ultrasound-mediated cascade-catalytic tumor ferroptosis. Angew Chem. Int. Ed. Engl. 2023;62(11) doi: 10.1002/anie.202217448. [DOI] [PubMed] [Google Scholar]
- 51.Gu Z., Zhong D., Hou X., Wei X., Liu C., Zhang Y., Duan Z., Gu Z., Gong Q., Luo K. Unraveling ros conversion through enhanced enzyme-like activity with copper-doped cerium oxide for tumor nanocatalytic therapy. Adv. Sci. 2024;11(11) doi: 10.1002/advs.202307154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li L., Cao S., Wu Z., Guo R., Xie L., Wang L., Tang Y., Li Q., Luo X., Ma L., Cheng C., Qiu L. Modulating electron transfer in vanadium-based artificial enzymes for enhanced ROS-catalysis and disinfection. Adv Mater. 2022;34(17) doi: 10.1002/adma.202108646. [DOI] [PubMed] [Google Scholar]
- 53.Huang H., Geng W., Wu X., Zhang Y., Xie L., Ma T., Cheng C. Spiky artificial peroxidases with V-O-Fe pair sites for combating antibiotic-resistant pathogens. Angew Chem. Int. Ed. Engl. 2024;63(1) doi: 10.1002/anie.202310811. [DOI] [PubMed] [Google Scholar]
- 54.Fan X., Wu X., Yang F., Wang L., Ludwig K., Ma L., Trampuz A., Cheng C., Haag R. A nanohook-equipped bionanocatalyst for localized near-infrared-enhanced catalytic bacterial disinfection. Angew Chem. Int. Ed. Engl. 2022;61(8) doi: 10.1002/anie.202113833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zheng Y., Liu W., Qin Z., Chen Y., Jiang H., Wang X. Mercaptopyrimidine-conjugated gold nanoclusters as nanoantibiotics for combating multidrug-resistant superbugs. Bioconjugate Chem. 2018;29(9):3094–3103. doi: 10.1021/acs.bioconjchem.8b00452. [DOI] [PubMed] [Google Scholar]
- 56.Cai T., Fang G., Tian X., Yin J.J., Chen C., Ge C. Optimization of antibacterial efficacy of noble-metal-based core-shell nanostructures and effect of natural organic matter. ACS Nano. 2019;13(11):12694–12702. doi: 10.1021/acsnano.9b04366. [DOI] [PubMed] [Google Scholar]
- 57.Hu W.C., Younis M.R., Zhou Y., Wang C., Xia X.H. In situ fabrication of ultrasmall gold nanoparticles/2D MOFs hybrid as nanozyme for antibacterial therapy. Small. 2020;16(23) doi: 10.1002/smll.202000553. [DOI] [PubMed] [Google Scholar]
- 58.Yao H., Zhou R., Wang J., Wei Y., Li S., Zhang Z., Du X.D., Wu S., Shi J. Pathogen-targeting bimetallic nanozymes as ultrasonic-augmented ROS generator against multidrug resistant bacterial infection. Adv Healthc Mater. 2023;12(25) doi: 10.1002/adhm.202300449. [DOI] [PubMed] [Google Scholar]
- 59.Shan J., Jin X., Zhang C., Huang M., Xing J., Li Q., Cui Y., Niu Q., Chen X.L., Wang X. Metal natural product complex Ru-procyanidins with quadruple enzymatic activity combat infections from drug-resistant bacteria. Acta Pharm. Sin. B. 2024;14(5):2298–2316. doi: 10.1016/j.apsb.2023.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chakraborty N., Gandhi S., Verma R., Roy I. Emerging prospects of nanozymes for antibacterial and anticancer applications. Biomedicines. 2022;10(6) doi: 10.3390/biomedicines10061378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu Q., Zhang A., Wang R., Zhang Q., Cui D. A review on metal- and metal oxide-based nanozymes: properties, mechanisms, and applications. Nano-Micro Lett. 2021;13(1):154. doi: 10.1007/s40820-021-00674-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oh M.J., Yoon S., Babeer A., Liu Y., Ren Z., Xiang Z., Miao Y., Cormode D.P., Chen C., Steager E., Koo H. Nanozyme-based robotics approach for targeting fungal infection. Adv Mater. 2024;36(10) doi: 10.1002/adma.202300320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xiao S., Xie L., Gao Y., Wang M., Geng W., Wu X., Rodriguez R.D., Cheng L., Qiu L., Cheng C. Artificial phages with biocatalytic spikes for synergistically eradicating antibiotic-resistant biofilms. Adv Mater. 2024;36(32) doi: 10.1002/adma.202404411. [DOI] [PubMed] [Google Scholar]
- 64.Li Y., Fu R., Duan Z., Zhu C., Fan D. Construction of multifunctional hydrogel based on the tannic acid-metal coating decorated MoS(2) dual nanozyme for bacteria-infected wound healing. Bioact. Mater. 2022;9:461–474. doi: 10.1016/j.bioactmat.2021.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu S., Bai Q., Jiang Y., Gao Y., Chen Z., Shang L., Zhang S., Yu L., Yang D., Sui N., Zhu Z. Multienzyme-like nanozyme encapsulated ocular microneedles for keratitis treatment. Small. 2024;20(21) doi: 10.1002/smll.202308403. [DOI] [PubMed] [Google Scholar]
- 66.Wang W., Cui Y., Wei X., Zang Y., Chen X., Cheng L., Wang X. CuCo(2)O(4) nanoflowers with multiple enzyme activities for treating bacterium-infected wounds via cuproptosis-like death. ACS Nano. 2024;18(24):15845–15863. doi: 10.1021/acsnano.4c02825. [DOI] [PubMed] [Google Scholar]
- 67.Han D., Liu X., Wu S. Metal organic framework-based antibacterial agents and their underlying mechanisms. Chem. Soc. Rev. 2022;51(16):7138–7169. doi: 10.1039/d2cs00460g. [DOI] [PubMed] [Google Scholar]
- 68.Hosseini Hooshiar M., Badkoobeh A., Kolahdouz S., Tadayonfard A., Mozaffari A., Nasiri K., Salari S., Safaralizadeh R., Yasamineh S. The potential use of nanozymes as an antibacterial agents in oral infection, periodontitis, and peri-implantitis. J. Nanobiotechnol. 2024;22(1):207. doi: 10.1186/s12951-024-02472-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang X., Li G., Wu D., Li X., Hu N., Chen J., Chen G., Wu Y. Recent progress in the design fabrication of metal-organic frameworks-based nanozymes and their applications to sensing and cancer therapy. Biosens. Bioelectron. 2019;137:178–198. doi: 10.1016/j.bios.2019.04.061. [DOI] [PubMed] [Google Scholar]
- 70.Falahati M., Sharifi M., Hagen T. Explaining chemical clues of metal organic framework-nanozyme nano-/micro-motors in targeted treatment of cancers: benchmarks and challenges. J. Nanobiotechnol. 2022;20(1):153. doi: 10.1186/s12951-022-01375-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Long Y., Li L., Xu T., Wu X., Gao Y., Huang J., He C., Ma T., Ma L., Cheng C., Zhao C. Hedgehog artificial macrophage with atomic-catalytic centers to combat Drug-resistant bacteria. Nat. Commun. 2021;12(1):6143. doi: 10.1038/s41467-021-26456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shuai C.J., Zan J., Deng F., Yang Y.W., Peng S.P., Zhao Z.Y. Core-shell-Structured ZIF-8@PDA-HA with controllable zinc ion release and superior bioactivity for improving a poly-L-lactic acid scaffold. ACS Sustain. Chem. Eng. 2021;9(4):1814–1825. [Google Scholar]
- 73.Hoop M., Walde C.F., Riccò R., Mushtaq F., Terzopoulou A., Chen X.Z., deMello A.J., Doonan C.J., Falcaro P., Nelson B.J., Puigmartí-Luis J., Pané S. Biocompatibility characteristics of the metal organic framework ZIF-8 for therapeutical applications. Appl. Mater. Today. 2018;11:13–21. [Google Scholar]
- 74.Wang M., Zhou X., Li Y., Dong Y., Meng J., Zhang S., Xia L., He Z., Ren L., Chen Z., Zhang X. Triple-synergistic MOF-nanozyme for efficient antibacterial treatment. Bioact. Mater. 2022;17:289–299. doi: 10.1016/j.bioactmat.2022.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu L., Luo Y., Wang C., Wu S., Zheng Y., Li Z., Cui Z., Liang Y., Zhu S., Shen J., Liu X. Self-driven electron transfer biomimetic enzymatic catalysis of bismuth-doped PCN-222 MOF for rapid therapy of bacteria-infected wounds. ACS Nano. 2023;17(2):1448–1463. doi: 10.1021/acsnano.2c10203. [DOI] [PubMed] [Google Scholar]
- 76.Liu C., Zhao X., Wang Z., Zhao Y., Li R., Chen X., Chen H., Wan M., Wang X. Metal-organic framework-modulated Fe(3)O(4) composite au nanoparticles for antibacterial wound healing via synergistic peroxidase-like nanozymatic catalysis. J. Nanobiotechnol. 2023;21(1):427. doi: 10.1186/s12951-023-02186-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Peng C., Pang R., Li J., Wang E. Current advances on the single-atom nanozyme and its bioapplications. Adv Mater. 2024;36(10) doi: 10.1002/adma.202211724. [DOI] [PubMed] [Google Scholar]
- 78.Liu Q., Liu X., He X., Wang D., Zheng C., Jin L., Shen J. Iron-single-atom nanozyme with NIR enhanced catalytic activities for facilitating MRSA-infected wound therapy. Adv. Sci. 2024;11(15) doi: 10.1002/advs.202308684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shen J., Chen J., Qian Y., Wang X., Wang D., Pan H., Wang Y. Atomic engineering of single-atom nanozymes for biomedical applications. Adv Mater. 2024;36(21) doi: 10.1002/adma.202313406. [DOI] [PubMed] [Google Scholar]
- 80.Zhang H., Wang P., Zhang J., Sun Q., He Q., He X., Chen H., Ji H. Boosting the catalase-like activity of SAzymes via facile tuning of the distances between neighboring atoms in single-iron sites. Angew Chem. Int. Ed. Engl. 2024;63(9) doi: 10.1002/anie.202316779. [DOI] [PubMed] [Google Scholar]
- 81.Qin T., Chen Y., Miao X., Shao M., Xu N., Mou C., Chen Z., Yin Y., Chen S., Yin Y., Gao L., Peng D., Liu X. Low-temperature adaptive single-atom iron nanozymes against viruses in the cold chain. Adv Mater. 2024;36(15) doi: 10.1002/adma.202309669. [DOI] [PubMed] [Google Scholar]
- 82.Bai J., Feng Y., Li W., Cheng Z., Rosenholm J.M., Yang H., Pan G., Zhang H., Geng D. vol. 6. Research (Wash D C); 2023. p. 31. (Alternative Copper-Based Single-Atom Nanozyme with Superior Multienzyme Activities and NIR-II Responsiveness to Fight against Deep Tissue Infections). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gao Z., Song Z., Guo R., Zhang M., Wu J., Pan M., Du Q., He Y., Wang X., Gao L., Jin Y., Jing Z., Zheng J. Mn single-atom nanozyme functionalized 3D-printed bioceramic scaffolds for enhanced antibacterial activity and bone regeneration. Adv Healthc Mater. 2024;13(13) doi: 10.1002/adhm.202303182. [DOI] [PubMed] [Google Scholar]
- 84.Li Z.P., Xu D.Q., Deng Z.A., Yin J.A., Qian Y.A., Hou J.T., Ding X., Shen J.L., He X.J. Single-Atom-Catalyzed MXene-Based nanoplatform with Photo-Enhanced Peroxidase-Like activity nanotherapeutics for Staphylococcus aureus infection. Chem. Eng. J. 2023;452 [Google Scholar]
- 85.Yang B., Chen Y., Shi J. Nanocatalytic Medicine. Adv Mater. 2019;31(39) doi: 10.1002/adma.201901778. [DOI] [PubMed] [Google Scholar]
- 86.Yu Q., Wang C., Zhang X., Chen H., Wu M.X., Lu M. Photochemical strategies toward precision targeting against multidrug-resistant bacterial infections. ACS Nano. 2024;18(22):14085–14122. doi: 10.1021/acsnano.3c12714. [DOI] [PubMed] [Google Scholar]
- 87.Ge M., Guo H., Zong M., Chen Z., Liu Z., Lin H., Shi J. Bandgap-engineered germanene nanosheets as an efficient photodynamic agent for cancer therapy. Angew Chem. Int. Ed. Engl. 2023;62(12) doi: 10.1002/anie.202215795. [DOI] [PubMed] [Google Scholar]
- 88.Feng P., He R., Gu Y., Yang F., Pan H., Shuai C. Construction of antibacterial bone implants and their application in bone regeneration. Mater. Horiz. 2024;11(3):590–625. doi: 10.1039/d3mh01298k. [DOI] [PubMed] [Google Scholar]
- 89.Yu Q., Li X., Wang J., Guo L., Huang L., Gao W. Recent advances in reprogramming strategy of tumor microenvironment for rejuvenating photosensitizers-mediated photodynamic therapy. Small. 2024;20(16) doi: 10.1002/smll.202305708. [DOI] [PubMed] [Google Scholar]
- 90.Wang Y., Staudinger J.N., Mindt T.L., Gasser G. Theranostics with photodynamic therapy for personalized medicine: to see and to treat. Theranostics. 2023;13(15):5501–5544. doi: 10.7150/thno.87363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yang J., Ren B., Yin X., Xiang L., Hua Y., Huang X., Wang H., Mao Z., Chen W., Deng J. Expanded ROS generation and hypoxia reversal: excipient-free self-assembled nanotheranostics for enhanced cancer photodynamic immunotherapy. Adv Mater. 2024 doi: 10.1002/adma.202402720. [DOI] [PubMed] [Google Scholar]
- 92.Guo L., Tian Y., Zhou L., Kang S., Zhang C., Liu W., Diao H., Feng L. Tailored phototherapy agent by infection site in situ activated against methicillin-resistant S. aureus. Adv Healthc Mater. 2024 doi: 10.1002/adhm.202400593. [DOI] [PubMed] [Google Scholar]
- 93.Yang M., Qiu S., Coy E., Li S., Załęski K., Zhang Y., Pan H., Wang G. NIR-responsive TiO(2) biometasurfaces: toward in situ photodynamic antibacterial therapy for biomedical implants. Adv Mater. 2022;34(6) doi: 10.1002/adma.202106314. [DOI] [PubMed] [Google Scholar]
- 94.Kang S., Park D.H., Hwang J. Hierarchical ZnO nano-spines grown on a carbon fiber seed layer for efficient VOC removal and airborne virus and bacteria inactivation. J. Hazard Mater. 2022;424(Pt A) doi: 10.1016/j.jhazmat.2021.127262. [DOI] [PubMed] [Google Scholar]
- 95.Zhou Z., Li B., Liu X., Li Z., Zhu S., Liang Y., Cui Z., Wu S. Recent progress in photocatalytic antibacterial. ACS Appl. Bio Mater. 2021;4(5):3909–3936. doi: 10.1021/acsabm.0c01335. [DOI] [PubMed] [Google Scholar]
- 96.Verma S.K., Jha E., Panda P.K., Thirumurugan A., Parashar S.K.S., Patro S., Suar M. Mechanistic insight into size-dependent enhanced cytotoxicity of industrial antibacterial titanium oxide nanoparticles on colon cells because of reactive oxygen species quenching and neutral lipid alteration. ACS Omega. 2018;3(1):1244–1262. doi: 10.1021/acsomega.7b01522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Verma S.K., Jha E., Panda P.K., Mukherjee M., Thirumurugan A., Makkar H., Das B., Parashar S.K.S., Suar M. Mechanistic insight into ROS and neutral lipid alteration induced toxicity in the human model with fins (Danio rerio) by industrially synthesized titanium dioxide nanoparticles. Toxicol. Res. 2018;7(2):244–257. doi: 10.1039/c7tx00300e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li Z., Li Z., Zuo C., Fang X. Application of nanostructured TiO(2) in UV photodetectors: a review. Adv Mater. 2022;34(28) doi: 10.1002/adma.202109083. [DOI] [PubMed] [Google Scholar]
- 99.Liao Y., Song J., Si Y., Yu J., Ding B. Superelastic and photothermal RGO/Zr-doped TiO(2) nanofibrous aerogels enable the rapid decomposition of chemical warfare agents. Nano Lett. 2022;22(11):4368–4375. doi: 10.1021/acs.nanolett.2c00776. [DOI] [PubMed] [Google Scholar]
- 100.Lu B., Zhang J., Zhu G., Liu T., Chen J., Liang X. Highly hydrophilic and dispersed TiO(2) nano-system with enhanced photocatalytic antibacterial activities and accelerated tissue regeneration under visible light. J. Nanobiotechnol. 2023;21(1):491. doi: 10.1186/s12951-023-02241-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kciuk M., Marciniak B., Mojzych M., Kontek R. Focus on UV-induced DNA damage and repair-disease relevance and protective strategies. Int. J. Mol. Sci. 2020;21(19) doi: 10.3390/ijms21197264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Weng Z., Wei Q., Ye C., Xu Y., Gao J., Zhang W., Liu L., Zhang Y., Hu J., Zhong Q., Sun J., Wang X. Traditional herb (moxa) modified zinc oxide nanosheets for quick, efficient and high tissue penetration therapy of fungal infection. ACS Nano. 2024;18(6):5180–5195. doi: 10.1021/acsnano.3c13164. [DOI] [PubMed] [Google Scholar]
- 103.Cheng H., Wang J., Yang Y., Shi H., Shi J., Jiao X., Han P., Yao X., Chen W., Wei X., Chu P.K., Zhang X. Ti(3) C(2) T(X) MXene modified with ZnTCPP with bacteria capturing capability and enhanced visible light photocatalytic antibacterial activity. Small. 2022;18(26) doi: 10.1002/smll.202200857. [DOI] [PubMed] [Google Scholar]
- 104.Wu Y., Li J., Zhu L., Wang D., Song J., Yu X., Li Y., Tang B.Z. Photosensitive AIEgens sensitize bacteria to oxidative damage and modulate the inflammatory responses of macrophages to salvage the photodynamic therapy against MRSA. Biomaterials. 2024;309 doi: 10.1016/j.biomaterials.2024.122583. [DOI] [PubMed] [Google Scholar]
- 105.Fang Y., Liu X., Guo H., Zhang Y., Wu H., Zhou X., Chen X., Qin H., Gao H., Liu Y. AIE bioconjugates for accurate identification and in vivo targeted treatment of bacterial infection based on bioorthogonal reaction. Adv Healthc Mater. 2023;12(24) doi: 10.1002/adhm.202300044. [DOI] [PubMed] [Google Scholar]
- 106.Zhu W., Li Y., Guo S., Guo W.J., Peng T., Li H., Liu B., Peng H.Q., Tang B.Z. Stereoisomeric engineering of aggregation-induced emission photosensitizers towards fungal killing. Nat. Commun. 2022;13(1):7046. doi: 10.1038/s41467-022-34358-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lochenie C., Duncan S., Zhou Y., Fingerhut L., Kiang A., Benson S., Jiang G., Liu X., Mills B., Vendrell M. Photosensitizer-amplified antimicrobial materials for broad-spectrum ablation of resistant pathogens in ocular infections. Adv Mater. 2024 doi: 10.1002/adma.202404107. [DOI] [PubMed] [Google Scholar]
- 108.Chen W., Ouyang J., Liu H., Chen M., Zeng K., Sheng J., Liu Z., Han Y., Wang L., Li J., Deng L., Liu Y.N., Guo S. Black phosphorus nanosheet-based drug delivery system for synergistic photodynamic/photothermal/chemotherapy of cancer. Adv Mater. 2017;29(5) doi: 10.1002/adma.201603864. [DOI] [PubMed] [Google Scholar]
- 109.Li Y., Liu C., Cheng X., Wang J., Pan Y., Liu C., Zhang S., Jian X. PDA-BPs integrated mussel-inspired multifunctional hydrogel coating on PPENK implants for anti-tumor therapy, antibacterial infection and bone regeneration. Bioact. Mater. 2023;27:546–559. doi: 10.1016/j.bioactmat.2023.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang C., Xiao Y., Zhu W., Chu J., Xu J., Zhao H., Shen F., Peng R., Liu Z. Photosensitizer-modified MnO(2) nanoparticles to enhance photodynamic treatment of abscesses and boost immune protection for treated mice. Small. 2020;16(28) doi: 10.1002/smll.202000589. [DOI] [PubMed] [Google Scholar]
- 111.Jiang F., Wang J., Ren Z., Hu Y., Wang B., Li M., Yu J., Tang J., Guo G., Cheng Y., Han P., Shen H. Targeted light-induced immunomodulatory strategy for implant-associated infections via reversing biofilm-mediated immunosuppression. ACS Nano. 2024;18(9):6990–7010. doi: 10.1021/acsnano.3c10172. [DOI] [PubMed] [Google Scholar]
- 112.Zhang A., Wu H., Chen X., Chen Z., Pan Y., Qu W., Hao H., Chen D., Xie S. Targeting and arginine-driven synergizing photodynamic therapy with nutritional immunotherapy nanosystems for combating MRSA biofilms. Sci. Adv. 2023;9(28) doi: 10.1126/sciadv.adg9116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhuang J., Qi G., Feng Y., Wu M., Zhang H., Wang D., Zhang X., Chong K.C., Li B., Liu S., Tian J., Shan Y., Mao D., Liu B. Thymoquinone as an electron transfer mediator to convert Type II photosensitizers to Type I photosensitizers. Nat. Commun. 2024;15(1):4943. doi: 10.1038/s41467-024-49311-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Iravani S. Nanophotocatalysts against viruses and antibiotic-resistant bacteria: recent advances. Crit. Rev. Microbiol. 2022;48(1):67–82. doi: 10.1080/1040841X.2021.1944053. [DOI] [PubMed] [Google Scholar]
- 115.Liang S., Yao J., Liu D., Rao L., Chen X., Wang Z. Harnessing nanomaterials for cancer sonodynamic immunotherapy. Adv Mater. 2023;35(33) doi: 10.1002/adma.202211130. [DOI] [PubMed] [Google Scholar]
- 116.Song X., Zhang Q., Chang M., Ding L., Huang H., Feng W., Xu T., Chen Y. Nanomedicine-enabled sonomechanical, sonopiezoelectric, sonodynamic, and sonothermal therapy. Adv Mater. 2023;35(31) doi: 10.1002/adma.202212259. [DOI] [PubMed] [Google Scholar]
- 117.Chang M., Zhang L., Wang Z., Chen L., Dong Y., Yang J., Chen Y. Nanomedicine/materdicine-enabled sonocatalytic therapy. Adv. Drug Deliv. Rev. 2024;205 doi: 10.1016/j.addr.2023.115160. [DOI] [PubMed] [Google Scholar]
- 118.Qin W., Yang Q., Zhu C., Jiao R., Lin X., Fang C., Guo J., Zhang K. A distinctive insight into inorganic sonosensitizers: design principles and application domains. Small. 2024;20(25) doi: 10.1002/smll.202311228. [DOI] [PubMed] [Google Scholar]
- 119.Li D., Yang Y., Li D., Pan J., Chu C., Liu G. Organic sonosensitizers for sonodynamic therapy: from small molecules and nanoparticles toward clinical development. Small. 2021;17(42) doi: 10.1002/smll.202101976. [DOI] [PubMed] [Google Scholar]
- 120.Zhou R., Chang M., Shen M., Cong Y., Chen Y., Wang Y. Sonocatalytic optimization of titanium-based therapeutic nanomedicine. Adv. Sci. 2023;10(25) doi: 10.1002/advs.202301764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li G., Wu S., Liu J., Wang K., Chen X., Liu H. Narrow bandgap Schottky heterojunction sonosensitizer with high electron-hole separation boosted sonodynamic therapy in bladder cancer. Adv Mater. 2024 doi: 10.1002/adma.202401252. [DOI] [PubMed] [Google Scholar]
- 122.Yang Z., Yuan M., Cheng Z., Liu B., Ma Z., Ma J., Zhang J., Ma X., Ma P., Lin J. Defect-repaired g-C(3)N(4) nanosheets: elevating the efficacy of sonodynamic cancer therapy through enhanced charge carrier migration. Angew Chem. Int. Ed. Engl. 2024;63(18) doi: 10.1002/anie.202401758. [DOI] [PubMed] [Google Scholar]
- 123.Liang C., Xie J., Luo S., Huang C., Zhang Q., Huang H., Zhang P. A highly potent ruthenium(II)-sonosensitizer and sonocatalyst for in vivo sonotherapy. Nat. Commun. 2021;12(1):5001. doi: 10.1038/s41467-021-25303-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang H., Guo J., Lin W., Fu Z., Ji X., Yu B., Lu M., Cui W., Deng L., Engle J.W., Wu Z., Cai W., Ni D. Open-Shell nanosensitizers for glutathione responsive cancer sonodynamic therapy. Adv Mater. 2022;34(15) doi: 10.1002/adma.202110283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhan M., Wang F., Liu Y., Zhou J., Zhao W., Lu L., Li J., He X. Dual-cascade activatable nanopotentiators reshaping adenosine metabolism for sono-chemodynamic-immunotherapy of deep tumors. Adv. Sci. 2023;10(10) doi: 10.1002/advs.202207200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhao P.H., Wu Y.L., Li X.Y., Feng L.L., Zhang L., Zheng B.Y., Ke M.R., Huang J.D. Aggregation-enhanced sonodynamic activity of phthalocyanine-artesunate conjugates. Angew Chem. Int. Ed. Engl. 2022;61(5) doi: 10.1002/anie.202113506. [DOI] [PubMed] [Google Scholar]
- 127.Liu K., Jiang Z., Zhao F., Wang W., Jäkle F., Wang N., Tang X., Yin X., Chen P. Triarylboron-doped acenethiophenes as organic sonosensitizers for highly efficient sonodynamic therapy with low phototoxicity. Adv Mater. 2022;34(49) doi: 10.1002/adma.202206594. [DOI] [PubMed] [Google Scholar]
- 128.Tian M., Li Y., Li Y., Yang T., Chen H., Guo J., Liu Y., Liu P. Sonodynamic therapy-driven immunotherapy: constructing AIE organic sonosensitizers using an advanced receptor-regulated strategy. Small. 2024 doi: 10.1002/smll.202400654. [DOI] [PubMed] [Google Scholar]
- 129.Liu X., Wang J., Wu Y., Wu M., Song J. Ultrasound activated probe for disease imaging and therapy In-Vivo. Adv. Drug Deliv. Rev. 2024;205 doi: 10.1016/j.addr.2023.115158. [DOI] [PubMed] [Google Scholar]
- 130.Nene L.C., Abrahamse H. Design consideration of phthalocyanines as sensitizers for enhanced sono-photodynamic combinatorial therapy of cancer. Acta Pharm. Sin. B. 2024;14(3):1077–1097. doi: 10.1016/j.apsb.2023.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sun D., Pang X., Cheng Y., Ming J., Xiang S., Zhang C., Lv P., Chu C., Chen X., Liu G., Zheng N. Ultrasound-switchable nanozyme augments sonodynamic therapy against multidrug-resistant bacterial infection. ACS Nano. 2020;14(2):2063–2076. doi: 10.1021/acsnano.9b08667. [DOI] [PubMed] [Google Scholar]
- 132.Xu K., Zou Y., Lin C., Zhang L., Tan M., Li M., Wu J., Li X., He Y., Liu P., Li K., Cai K. Cascade catalysis nanozyme for interfacial functionalization in combating implant infections associated with diabetes via sonodynamic therapy and adaptive immune activation. Biomaterials. 2024;311 doi: 10.1016/j.biomaterials.2024.122649. [DOI] [PubMed] [Google Scholar]
- 133.Lin H., Yang C., Luo Y., Ge M., Shen H., Zhang X., Shi J. Biomimetic nanomedicine-triggered in situ vaccination for innate and adaptive immunity activations for bacterial osteomyelitis treatment. ACS Nano. 2022;16(4):5943–5960. doi: 10.1021/acsnano.1c11132. [DOI] [PubMed] [Google Scholar]
- 134.He C.N., Feng P.P., Hao M.M., Tang Y., Wu X., Cui W.G., Ma J.Y., Ke C.H. Nanomaterials in antibacterial photodynamic therapy and antibacterial sonodynamic therapy. Adv. Funct. Mater. 2024 [Google Scholar]
- 135.He X.J., Hou J.T., Sun X.S., Jangili P., An J.S., Qian Y.N., Kim J.S., Shen J.L. NIR-II photo-amplified sonodynamic therapy using sodium molybdenum bronze nanoplatform against subcutaneous Staphylococcus aureus infection. Adv. Funct. Mater. 2022;32(38) [Google Scholar]
- 136.Cao X., Li M., Liu Q., Zhao J., Lu X., Wang J. Inorganic sonosensitizers for sonodynamic therapy in cancer treatment. Small. 2023;19(42) doi: 10.1002/smll.202303195. [DOI] [PubMed] [Google Scholar]
- 137.Qian Y., Wang J., Geng X., Jia B., Wang L., Li Y.Q., Geng B., Huang W. Graphene quantum dots nanoantibiotic-sensitized TiO(2-) (x) heterojunctions for sonodynamic-nanocatalytic therapy of multidrug-resistant bacterial infections. Adv Healthc Mater. 2024 doi: 10.1002/adhm.202400659. [DOI] [PubMed] [Google Scholar]
- 138.Zeng J., Gu C., Geng X., Lin K., Xie Y., Chen X. Combined photothermal and sonodynamic therapy using a 2D black phosphorus nanosheets loaded coating for efficient bacterial inhibition and bone-implant integration. Biomaterials. 2023;297 doi: 10.1016/j.biomaterials.2023.122122. [DOI] [PubMed] [Google Scholar]
- 139.Guan S., Xu W., Tan J., Zhang X., Liu X., Liu L., Qian S., Hou Z., Zhu H., Qiu J., Yeung K.W.K., Zheng Y., Liu X. Metainterface heterostructure enhances sonodynamic therapy for disrupting secondary biofilms. ACS Nano. 2024;18(23):15114–15129. doi: 10.1021/acsnano.4c02605. [DOI] [PubMed] [Google Scholar]
- 140.Ku M., Mao C., Wu S., Zheng Y., Li Z., Cui Z., Zhu S., Shen J., Liu X. Lattice strain engineering of Ti(3)C(2) narrows band gap for realizing extraordinary sonocatalytic bacterial killing. ACS Nano. 2023;17(15):14840–14851. doi: 10.1021/acsnano.3c03134. [DOI] [PubMed] [Google Scholar]
- 141.Yang F., Dong J., Li Z., Wang Z. Metal-organic frameworks (MOF)-Assisted sonodynamic therapy in anticancer applications. ACS Nano. 2023;17(5):4102–4133. doi: 10.1021/acsnano.2c10251. [DOI] [PubMed] [Google Scholar]
- 142.Meng X., Sun S., Gong C., Yang J., Yang Z., Zhang X., Dong H. Ag-doped metal-organic frameworks’ heterostructure for sonodynamic therapy of deep-seated cancer and bacterial infection. ACS Nano. 2022;17(2):1174–1186. doi: 10.1021/acsnano.2c08687. [DOI] [PubMed] [Google Scholar]
- 143.Zeng Y., Ouyang Q., Yu Y., Tan L., Liu X., Zheng Y., Wu S. Defective homojunction porphyrin-based metal-organic frameworks for highly efficient sonodynamic therapy. Small Methods. 2023;7(1) doi: 10.1002/smtd.202201248. [DOI] [PubMed] [Google Scholar]
- 144.Xu Y., Pang Y., Luo L., Sharma A., Yang J., Li C., Liu S., Zhan J., Sun Y. De novo designed Ru(II) metallacycle as a microenvironment-adaptive sonosensitizer and sonocatalyst for multidrug-resistant biofilms eradication. Angew Chem. Int. Ed. Engl. 2024;63(15) doi: 10.1002/anie.202319966. [DOI] [PubMed] [Google Scholar]
- 145.Gu T., Wang Y., Lu Y., Cheng L., Feng L., Zhang H., Li X., Han G., Liu Z. Platinum nanoparticles to enable electrodynamic therapy for effective cancer treatment. Adv Mater. 2019;31(14) doi: 10.1002/adma.201806803. [DOI] [PubMed] [Google Scholar]
- 146.Chen T., Gu T., Cheng L., Li X., Han G., Liu Z. Porous Pt nanoparticles loaded with doxorubicin to enable synergistic Chemo-/Electrodynamic Therapy. Biomaterials. 2020;255 doi: 10.1016/j.biomaterials.2020.120202. [DOI] [PubMed] [Google Scholar]
- 147.Lu Z., Gao J., Fang C., Zhou Y., Li X., Han G. Porous Pt nanospheres incorporated with GOx to enable synergistic oxygen-inductive starvation/electrodynamic tumor therapy. Adv. Sci. 2020;7(17) doi: 10.1002/advs.202001223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Qiao Z., Zhang K., Liu J., Cheng D., Yu B., Zhao N., Xu F.J. Biomimetic electrodynamic nanoparticles comprising ginger-derived extracellular vesicles for synergistic anti-infective therapy. Nat. Commun. 2022;13(1):7164. doi: 10.1038/s41467-022-34883-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Duan M., Huang C., Zhang G., Shi H., Zhang P., Li L., Xu T., Zhao Z., Fu Z., Han J., Xu Y., Ding X. Spin-state conversion by asymmetrical orbital hybridization in Ni-doped Co(3) O(4) to boost singlet oxygen generation for microbial disinfection. Angew Chem. Int. Ed. Engl. 2024;63(12) doi: 10.1002/anie.202318924. [DOI] [PubMed] [Google Scholar]
- 150.Zhou K., Chigan D., Xu L., Liu C., Ding R., Li G., Zhang Z., Pei D., Li A., Guo B., Yan X., He G. Anti-sandwich structured photo-electronic wound dressing for highly efficient bacterial infection therapy. Small. 2021;17(33) doi: 10.1002/smll.202101858. [DOI] [PubMed] [Google Scholar]
- 151.Wang Z.L., Song J. Piezoelectric nanogenerators based on zinc oxide nanowire arrays. Science. 2006;312(5771):242–246. doi: 10.1126/science.1124005. [DOI] [PubMed] [Google Scholar]
- 152.Jing L., Zhuang F., Feng W., Huang H., Chen Y., Huang B. Doping-engineered piezoelectric ultrathin nanosheets for synergistically piezo-chemocatalytic antitumor and antibacterial therapies against cutaneous melanoma. Small. 2024 doi: 10.1002/smll.202401171. [DOI] [PubMed] [Google Scholar]
- 153.Fan Y., Ye J., Kang Y., Niu G., Shi J., Yuan X., Li R., Han J., Ji X. Biomimetic piezoelectric nanomaterial-modified oral microrobots for targeted catalytic and immunotherapy of colorectal cancer. Sci. Adv. 2024;10(19) doi: 10.1126/sciadv.adm9561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Montorsi M., Pucci C., De Pasquale D., Marino A., Ceccarelli M.C., Mazzuferi M., Bartolucci M., Petretto A., Prato M., Debellis D., De Simoni G., Pugliese G., Labardi M., Ciofani G. Ultrasound-activated piezoelectric nanoparticles trigger microglia activity against glioblastoma cells. Adv Healthc Mater. 2024 doi: 10.1002/adhm.202304331. [DOI] [PubMed] [Google Scholar]
- 155.Lei C., Lei J., Zhang X., Wang H., He Y., Zhang W., Tong B., Yang C., Feng X. Heterostructured piezocatalytic nanoparticles with enhanced ultrasound response for efficient repair of infectious bone defects. Acta Biomater. 2023;172:343–354. doi: 10.1016/j.actbio.2023.10.006. [DOI] [PubMed] [Google Scholar]
- 156.Chen Y., Wang C., Zhang Z., Yu F., Wang Y., Ding J., Zhao Z., Liu Y. 3D-printed piezocatalytic hydrogels for effective antibacterial treatment of infected wounds. Int. J. Biol. Macromol. 2024;268(Pt 2) doi: 10.1016/j.ijbiomac.2024.131637. [DOI] [PubMed] [Google Scholar]
- 157.Wang S.Y., Qiao Y.Q., Liu X.M., Zhu S.L., Zheng Y.F., Jiang H., Zhang Y., Shen J., Li Z.Y., Liang Y.Q., Cui Z.D., Chu P.K., Wu S.L. Reduced graphene oxides modified Bi2Te3 nanosheets for rapid photo-thermoelectric catalytic therapy of bacteria-infected wounds. Adv. Funct. Mater. 2023;33(3) [Google Scholar]
- 158.Arciola C.R., Campoccia D., Montanaro L. Implant infections: adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018;16(7):397–409. doi: 10.1038/s41579-018-0019-y. [DOI] [PubMed] [Google Scholar]
- 159.Zhang J., Pan Y., Liu L., Xu Y., Zhao C., Liu W., Rao L. Genetically edited cascade nanozymes for cancer immunotherapy. ACS Nano. 2024;18(19):12295–12310. doi: 10.1021/acsnano.4c01229. [DOI] [PubMed] [Google Scholar]
- 160.Zhao C., Wang C., Shan W., Wang Z., Chen X., Deng H. Nanomedicines for an enhanced immunogenic cell death-based in situ cancer vaccination response. Acc. Chem. Res. 2024;57(6):905–918. doi: 10.1021/acs.accounts.3c00771. [DOI] [PubMed] [Google Scholar]
- 161.Yang Y., Huang J., Liu M., Qiu Y., Chen Q., Zhao T., Xiao Z., Yang Y., Jiang Y., Huang Q., Ai K. Emerging sonodynamic therapy-based nanomedicines for cancer immunotherapy. Adv. Sci. 2023;10(2) doi: 10.1002/advs.202204365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mei J., Xu D., Wang L., Kong L., Liu Q., Li Q., Zhang X., Su Z., Hu X., Zhu W., Ye M., Wang J., Zhu C. Biofilm microenvironment-responsive self-assembly nanoreactors for all-stage biofilm associated infection through bacterial cuproptosis-like death and macrophage Re-rousing. Adv Mater. 2023;35(36) doi: 10.1002/adma.202303432. [DOI] [PubMed] [Google Scholar]
- 163.Li Q., Liu Q., Wang Z., Zhang X., Ma R., Hu X., Mei J., Su Z., Zhu W., Zhu C. Biofilm homeostasis interference therapy via (1) O(2) -sensitized hyperthermia and immune microenvironment Re-rousing for biofilm-associated infections elimination. Small. 2023;19(22) doi: 10.1002/smll.202300592. [DOI] [PubMed] [Google Scholar]
- 164.Thanabalasuriar A., Scott B.N.V., Peiseler M., Willson M.E., Zeng Z., Warrener P., Keller A.E., Surewaard B.G.J., Dozier E.A., Korhonen J.T., Cheng L.I., Gadjeva M., Stover C.K., DiGiandomenico A., Kubes P. Neutrophil extracellular traps confine Pseudomonas aeruginosa ocular biofilms and restrict brain invasion. Cell Host Microbe. 2019;25(4):526–536.e4. doi: 10.1016/j.chom.2019.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bhattacharya M., Berends E.T.M., Chan R., Schwab E., Roy S., Sen C.K., Torres V.J., Wozniak D.J. Staphylococcus aureus biofilms release leukocidins to elicit extracellular trap formation and evade neutrophil-mediated killing. Proc Natl Acad Sci U S A. 2018;115(28):7416–7421. doi: 10.1073/pnas.1721949115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Alhede M., Alhede M., Qvortrup K., Kragh K.N., Jensen P., Stewart P.S., Bjarnsholt T. The origin of extracellular DNA in bacterial biofilm infections in vivo. Pathog Dis. 2020;78(2) doi: 10.1093/femspd/ftaa018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Guo G., Liu Z., Yu J., You Y., Li M., Wang B., Tang J., Han P., Wu J., Shen H. Neutrophil function conversion driven by immune switchpoint regulator against diabetes-related biofilm infections. Adv Mater. 2024;36(8) doi: 10.1002/adma.202310320. [DOI] [PubMed] [Google Scholar]
- 168.Xu D., Hu J., Mei J., Zhou J., Wang Z., Zhang X., Liu Q., Su Z., Zhu W., Liu H., Zhu C. Nanoadjuvant-triggered STING activation evokes systemic immunotherapy for repetitive implant-related infections. Bioact. Mater. 2024;35:82–98. doi: 10.1016/j.bioactmat.2024.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yang C., Luo Y., Shen H., Ge M., Tang J., Wang Q., Lin H., Shi J., Zhang X. Inorganic nanosheets facilitate humoral immunity against medical implant infections by modulating immune co-stimulatory pathways. Nat. Commun. 2022;13(1):4866. doi: 10.1038/s41467-022-32405-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
No data was used for the research described in the article.