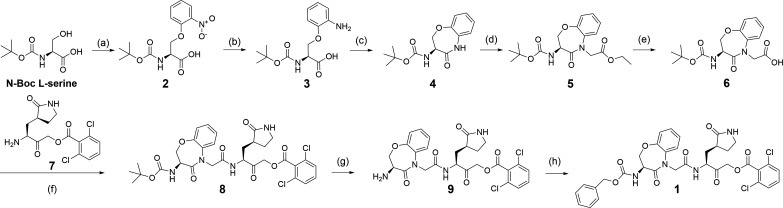
Fig 4.
Synthesis of compound 1. Reagents and conditions: (a) NaH, DMF, 1-fluoro-2-nitrobenzene, 0°C–40°C, 2 h. (b) 10% Pd/C, H2(g), EtOH, room temperature, 16 h. (c) 50% T3P in CH2Cl2, DIPEA, −20°C to 0°C, 1 h. (d) LiHMDS, THF, ethyl 2-bromoacetate, −78°C to room temperature, 12 h. (e) NaOH, THF: MeOH: H2O, 0°C to room temperature, 12 h. (f) 50% T3P in CH2Cl2, DIPEA, −0°C to room temperature, 1 h. (S)-3-amino-2-oxo-4-((S)-2-oxopyrrolidin-3-yl)butyl 2,6-dichloro benzoate. (g) 20% TFA in CH2Cl2, 0°C to room temperature, 2 h. (h) Cbz chloride, Et3N, CH2Cl2, 0°C to room temperature, 24 h.