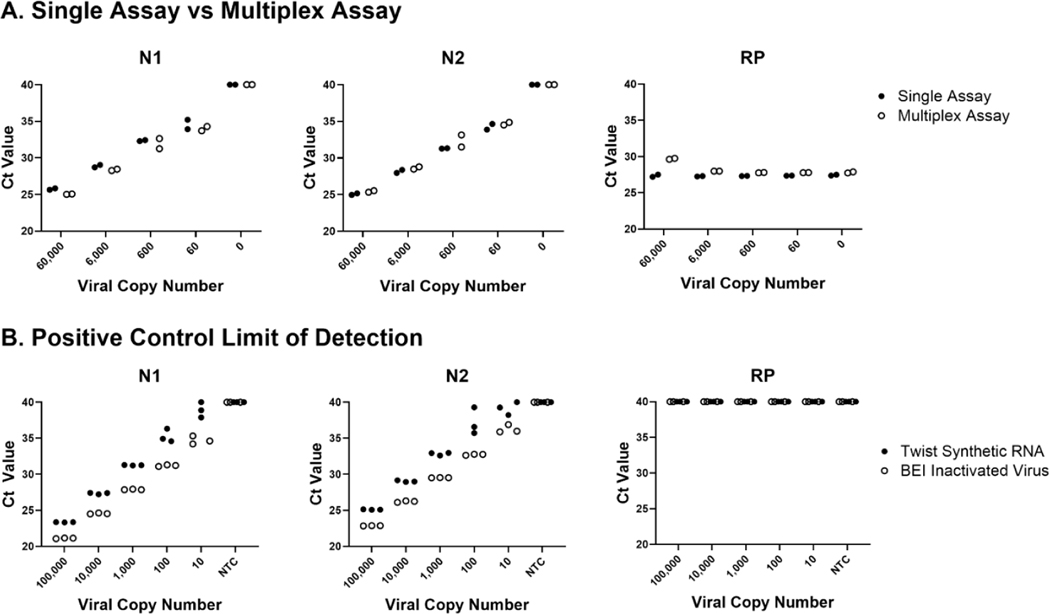
Figure 1. Development of Multiplex SARS-CoV-2 Assay.
Our SARS-CoV-2 multiplex assay was developed based on the primer/probe set recommended by the CDC for ease of obtaining Emergency Use Authorization if needed. A. The primer/probe sets utilized show similar sensitivity for viral nucleocapsid targets (N1 and N2) and human control target (RP) over a dilution series of positive control SARS-CoV-2 nucleocapsid plasmid with a background of 30,000 copies/μL RP plasmid. B. Repeat testing of the multiplex assay with more physiologically relevant positive controls including synthetic RNA (Twist Biosciences) and heat-inactivated virus (ATCC) shows sensitive detection of viral genetic material over a dilution series. Of note, we observed Twist synthetic RNA degrades faster in solution than inactive viral samples, which may account for the slightly higher Ct values shown.