Abstract
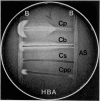
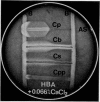
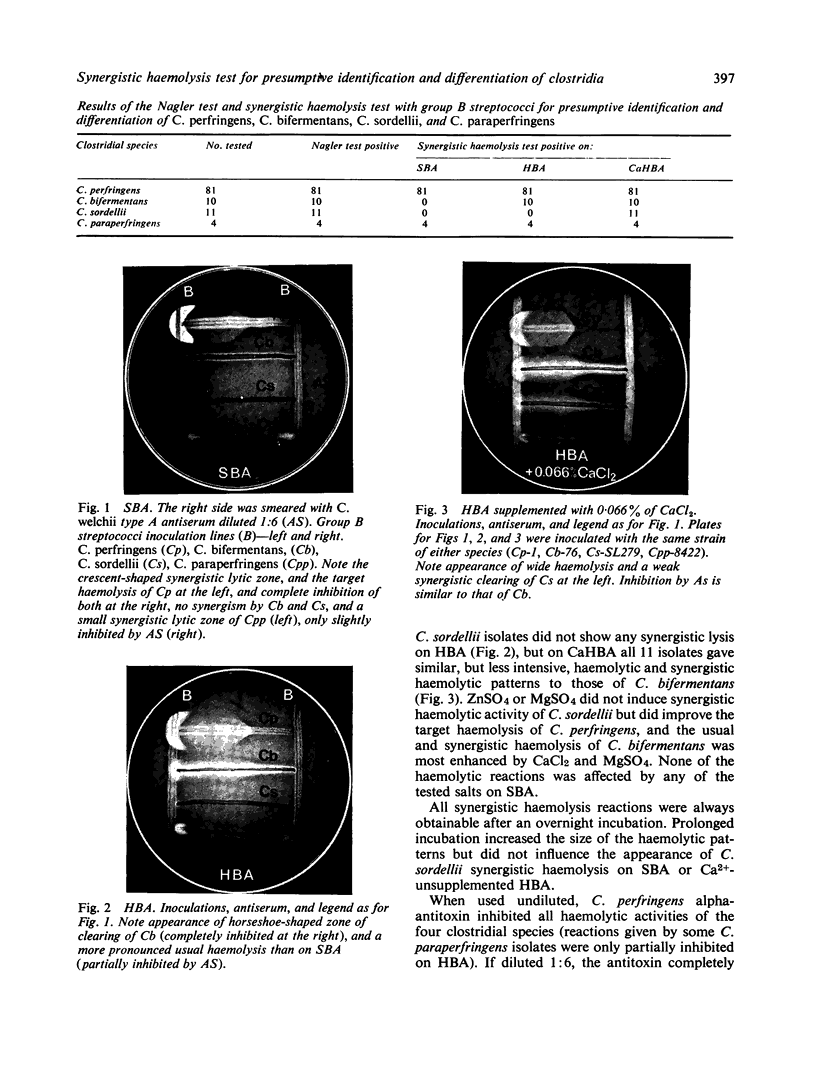
A new test for the presumptive identification of Clostridium perfringens, C. bifermentans, C. sordellii, and C. paraperfringens is described. The test is based on the synergistic haemolysis shown by the clostridia and group B streptococci on sheep and human and CaCl2-supplemented human blood agar. C. perfringens gave crescent-shaped synergistic lytic zones (7 to over 20 mm in length), and C. paraperfringens usually small-sized (3 mm), bullet-shaped reactions on all three types of media. C. bifermentans showed a horseshoe-shaped synergistic reaction only on human blood containing media, and C. sordellii only on CaCl2-supplemented human blood agar. C. perfringens type A antiserum inhibited synergistic lytic activities of the four species. The test provided a reliable method for presumptive identification and differentiation of the four clostridial species and may obviate the need for the Nagler test.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Colley C. M., Zwaal R. F., Roelofsen B., van Deenen L. L. Lytic and non-lytic degradation of phospholipids in mammalian erythrocytes by pure phospholipases. Biochim Biophys Acta. 1973 Apr 25;307(1):74–82. doi: 10.1016/0005-2736(73)90026-6. [DOI] [PubMed] [Google Scholar]
- Gubash S. M. Synergistic hemolysis phenomenon shown by an alpha-toxin-producing Clostridium perfingens and streptococcal CAMP factor in presumptive streptococcal grouping. J Clin Microbiol. 1978 Nov;8(5):480–488. doi: 10.1128/jcm.8.5.480-488.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEWIS G. M., MACFARLANE M. G. The lecithinase of Clostridium bifermentans toxin. Biochem J. 1953 Apr;54(1):138–142. doi: 10.1042/bj0540138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low D. K., Freer J. H., Arbuthnott J. P., Möllby R., Wadström T. Consequences of spingomyelin degradation in erythrocyte ghost membranes by staphylococcal beta-toxin (sphingomyelinase C). Toxicon. 1974 May;12(3):279–285. doi: 10.1016/0041-0101(74)90070-1. [DOI] [PubMed] [Google Scholar]
- Macfarlane M. G., Knight B. C. The biochemistry of bacterial toxins: The lecithinase activity of Cl. welchii toxins. Biochem J. 1941 Sep;35(8-9):884–902. doi: 10.1042/bj0350884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung L. S., Toabe R. The Egg Yolk Plate Reaction for the Presumptive Diagnosis of Clostridium sporogenes and Certain Species of the Gangrene and Botulinum Groups. J Bacteriol. 1947 Feb;53(2):139–147. doi: 10.1128/jb.53.2.139-147.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson G. J. Lipid composition of erythrocytes in various mammalian species. Biochim Biophys Acta. 1967 Oct 2;144(2):221–232. doi: 10.1016/0005-2760(67)90152-x. [DOI] [PubMed] [Google Scholar]
- Pastan I., Macchia V., Katzen R. A phospholipase specific for sphingomyelin from Clostridium perfringens. J Biol Chem. 1968 Jul 10;243(13):3750–3755. [PubMed] [Google Scholar]
- Sato H., Murata R. Role of zinc in the production of Clostridium perfringens alpha toxin. Infect Immun. 1973 Sep;8(3):360–369. doi: 10.1128/iai.8.3.360-369.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIS A. T., GOWLAND G. Some observations on the mechanism of the Nagler reaction. J Pathol Bacteriol. 1962 Jan;83:219–226. [PubMed] [Google Scholar]
- WILLIS A. T., HOBBS G. A medium for the identification of clostridia producing opalescence in egg-yolk emulsions. J Pathol Bacteriol. 1958 Apr;75(2):299–305. doi: 10.1002/path.1700750208. [DOI] [PubMed] [Google Scholar]
- Williams J., Wilkins A. G. Letter: On the evidence for partial gene duplication from amino acid sequence of bovine glutamate dehydrogenase. Nature. 1974 Feb 22;247(5442):556–557. doi: 10.1038/247556a0. [DOI] [PubMed] [Google Scholar]