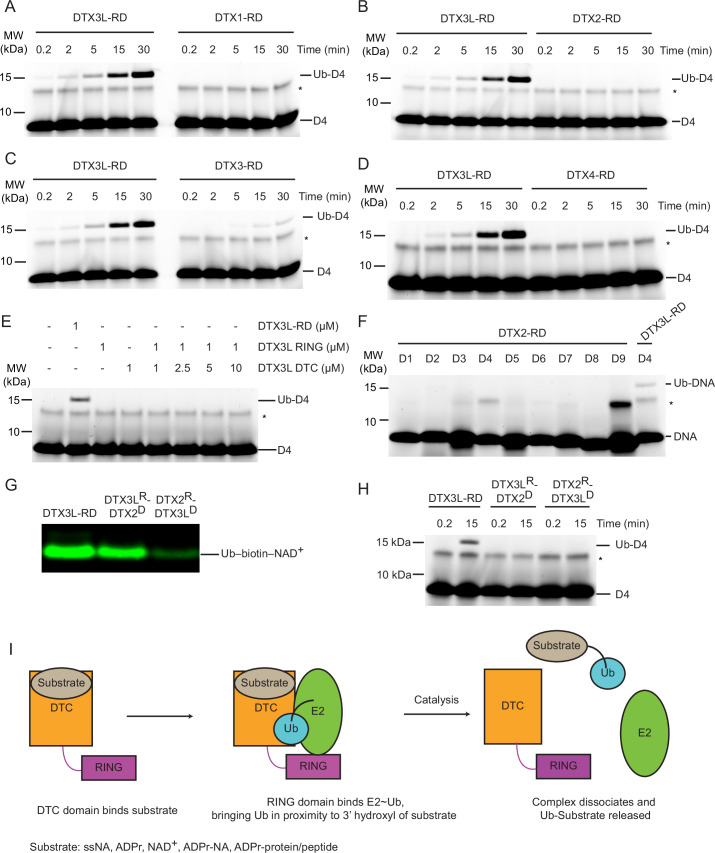
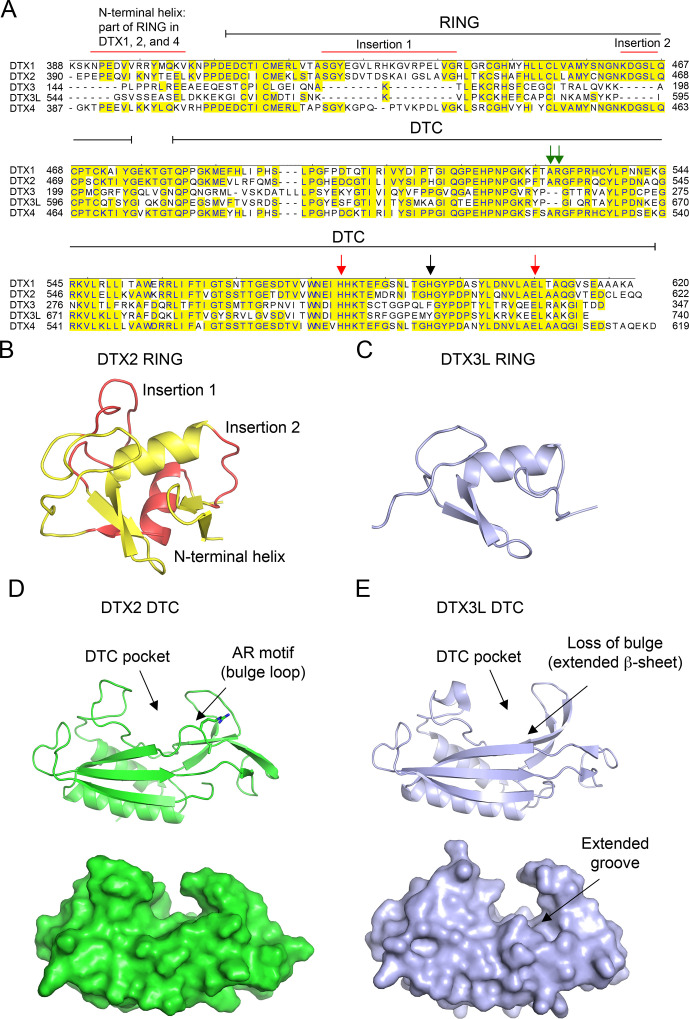
Figure 5. Select DTX RING-DTC domains catalyse ubiquitination of ssDNA.
(A) Fluorescently detected SDS-PAGE gel of in vitro ubiquitination of 6-FAM-labelled ssDNA D4 by DTX3L-RD or DTX1-RD in the presence of E1, UBE2D2, Ub, Mg2+-ATP. (B) As in (A) but with DTX2-RD. (C) As in (A) but with DTX3-RD. (D) As in (A) but with DTX4-RD. (E) As in (A) but with DTX3L-RD or DTX3L RING with increasing concentrations of DTX3L DTC. (F) Fluorescently detected SDS-PAGE gel of in vitro ubiquitination of 6-FAM-labelled ssDNA D1-9 by DTX2-RD. A reaction with DTX3L-RD and 6-FAM-labelled ssDNA D4 was included as a positive control. (G) Western blot of in vitro ubiquitination of biotin-NAD+ in the presence of E1, UBE2D2, Ub, Mg2+-ATP, NAD+, biotin-NAD+ with either DTX3L-RD, DTX3LR-DTX2D, or DTX2R-DTX3LD and separated by SDS-PAGE. (H) Fluorescently detected SDS-PAGE gel of in vitro ubiquitination of 6-FAM-labelled ssDNA D4 by DTX3L-RD, DTX3LR-DTX2D, or DTX2R-DTX3LD in the presence of E1, UBE2D2, Ub, Mg2+-ATP. (I) Schematic diagrams showing the proposed mechanism of ubiquitination of substrates by DTX3L-RD. Asterisks in (A–F) and (H) indicate contaminant bands from ssDNA. Raw unedited and uncropped gel images of (A–H) are shown in Figure 5—source data 1 and 2, respectively.