Abstract
Soybean dwarf virus (SbDV; family Tombusviridae, genus Luteovirus, species Luteovirus glycinis) is an RNA plant virus that is transmitted solely by aphids in a persistent, circulative and non-propagative manner. SbDV causes significant losses in cultivated Fabaceae, especially in subterranean clover (Trifolium subterraneum) pastures of mainland Australia. SbDV isolates are classified into four phenotypically distinguishable strains: YP, YS, DP, and DS. Y and D strains differ primarily in their host range, and P and S strains in their primary vector species. Genetically, Y and D strains separate into two clades in every genomic region except for the N-terminal region of the readthrough domain (N-RTD), in which P and S strains separate. SbDV diversity in Australia has yet to be investigated, so in this study, 41 isolates were collected from six different host species across two production regions of Australia: the south coast of Western Australia (‘south-west’) and northern New South Wales/southern Queensland (‘north-east’). A near-complete genome sequence of each isolate was obtained, and together with all 50 whole-genome sequences available in the GenBank database, underwent phylogenetic analysis of the whole genome nt and the N-RTD aa sequences. At the whole-genome level, the isolates separated into D and Y clades. At the N-RTD level, most of the isolates separated into P and S clades. All south-west isolates and 11 of the 31 north-east isolates were in the Y clade, and the remaining 20 north-east isolates were in the D clade. Except for one isolate that fell outside the P and S clades, all south-west and north-east isolates were in the P clade, suggesting that they are transmitted by Acyrthosiphon pisum and Myzus persicae. Available biological data largely supported the phenotypic inferences made from the phylogenetic analysis, suggesting that genetic data can provide critical epidemiological insights, provided that sufficient biological data have been collected.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00705-024-06142-z.
Introduction
Soybean dwarf virus (SbDV), currently classified as a member of the species Luteovirus glycinis in the genus Luteovirus of the family Tombusviridae [54], primarily infects members of the family Fabaceae and is transmitted by aphids in a persistent, circulative, and non-propagative manner [48]. SbDV causes serious disease in economically important grain and pasture legumes worldwide. In Australia, SbDV frequently causes leaf-reddening, severe stunting, and, occasionally, pasture collapse of subterranean clover (Trifolium subterraneum) [20, 29, 30, 33], which is an integral component of the pasture feed base of Australia’s $12.3 billion wool, dairy, and red meat production industries [39]. The most recent SbDV epidemic occurred on the south coast of south-west Western Australia (WA) in 2017 [44]. SbDV also infects many other important pasture legumes, including other clover species (Trifolium sp.), annual medics (Medicago spp.), French serradella (Ornithopus sativus), and biserrula (Biserrula pelecinus) without causing obvious disease [6, 28]. The importance and risk of SbDV to Australia’s $2 to 3 billion grain legume industry is less well understood, but the virus can cause severe disease in key species such as chickpea (Cicer arietinum), field pea (Pisum sativum), faba bean (Vicia faba), and lentil (Lens culinaris) [6, 36]. In the 2013 season in northern New South Wales (NSW), SbDV was responsible for >75% of the virus-infected chickpea plants [46]. In that season, the incidence of virus infection was generally less than 5%, but it was as high as 30-50% in several crops, suggesting that, in some seasons, SbDV may be a significant contributor to disease in grain legumes.
SbDV isolates are categorised into four strains: YP, YS, DP, and DS, distinguishable by epidemiologically important phenotypes. Yellowing (Y) and dwarfing (D) strains were initially divided based on their symptom expression in soybean (Glycine max) [48], and further research showed that they had different host ranges; only Y strain isolates infected white clover (T. repens), albus lupin (Lupinus albus), and common bean (Phaseolus vulgaris), and only D strain isolates infected red clover (T. pratense) [7, 21, 40, 49]. However, there is evidence that some host range indicators are not strict. For example, eastern USA D strain isolates can infect white clover [45]. Several other species or cultivars may also be strain-specific hosts or differ in susceptibility and sensitivity to different strains [7, 28]. P (pisum) strains are transmitted most efficiently by Acyrthosiphon pisum Harris (pea aphid) [3, 33, 55], and S (solani) strains are transmitted most efficiently by Aulacorthum solani Kaltenbach (foxglove aphid) [50]. Myzus persicae Sulzer (green peach aphid) and Aphis craccivora Koch (cowpea aphid) also transmit P strain isolates [6, 8, 17, 45], and several other vector species have possible virus strain specificity [8, 19, 21, 22, 28, 45, 55].
SbDV isolates have a ~5.7- to 5.9-kb positive-sense RNA genome containing five open reading frames (ORFs), some of which overlap [32]. Y and D strain isolates form separate clades when analysed at almost every region of the genome [45, 47, 50]. A recent study identified three Y subclades and two D subclades when analysing a global phylogeny of complete SbDV genome sequences [47]. Isolates form P and S strain clades when analysing the N-terminal region of the readthrough domain (N-RTD, encoded by ORF5), which plays a key role in aphid vector transmission and specificity [47, 51]. Stone et al. [47] found an N-RTD recombinant (MD2-Y) with a P strain phenotype and thus identified 12 amino acid (aa) positions that could determine vector specificity. Furthermore, they found that the majority of SbDV sequences fall into the P clade, suggesting that Ac. pisum-transmitted strains are the most widespread globally.
By using the relationship between genotype and phenotype, Stone et al. [47] analysed sequences obtained from 17 eastern USA field isolates to help assess the present and future risk of SbDV to USA soybean production. There has been no substantial phylogenetic analysis of Australian SbDV isolates undertaken to understand viral diversity in production regions impacted by SbDV. In the phylogeny reported by Stone et al. [47], two previously sequenced isolates from WA and one from NSW fell into the Y and P clades. SbDV isolate Tas-1 from the island state of Tasmania has been included in two genetic studies and on each occasion fell into the Y and S clades [47, 51], which is supported by the available phenotype data for this and other Tasmanian isolates, suggesting that it is a common strain in this region [19, 25, 28]. The few studies to have examined the phenotype of mainland Australian SbDV isolates also suggest that multiple strains are present. The first Australian report of SbDV in Victoria in 1970 included an isolate that was transmitted by Au. solani but not by M. persicae, suggesting that it was an S strain isolate [33]. However, it is unclear whether the isolate was a D or Y strain, as it was able to infect red clover, white clover, and common bean. Helms et al. [19] tested two isolates from south-east NSW and isolate Tas-1, which were transmitted by Au. solani but not Ac. pisum or M. persicae. One isolate (WA-8) obtained during a severe epidemic in subterranean clover pastures growing on the south coast of WA was transmitted by A. pisum at high efficiency and by M. persicae at lower efficiency and infected white clover, common bean, and albus lupin, but not red clover, suggesting it was a YP strain. SbDV has been found infecting white clover in WA, South Australia, NSW, Victoria, and Tasmania, suggesting that Y strain isolates are prevalent in southern winter-rainfall-dominant locations [37, 38, 41]. Therefore, based on the genetic and biological evidence available to date, it is likely that at least YS and YP strains are present in mainland Australia. In this study, we sequenced 41 SbDV isolates collected from 2013 to 2022 from various grain and pasture legume species growing in two geographically distinct production regions of Australia, expanding our understanding of SbDV genetic diversity in mainland Australia. We then performed phylogenetic analysis of these sequences together with all 50 complete or near-complete SbDV genome sequences available in the GenBank database. Using available phenotype data and the relationship between SbDV genotype and phenotype, we provide the first assessment of SbDV diversity in Australia.
Materials and methods
Isolate collection
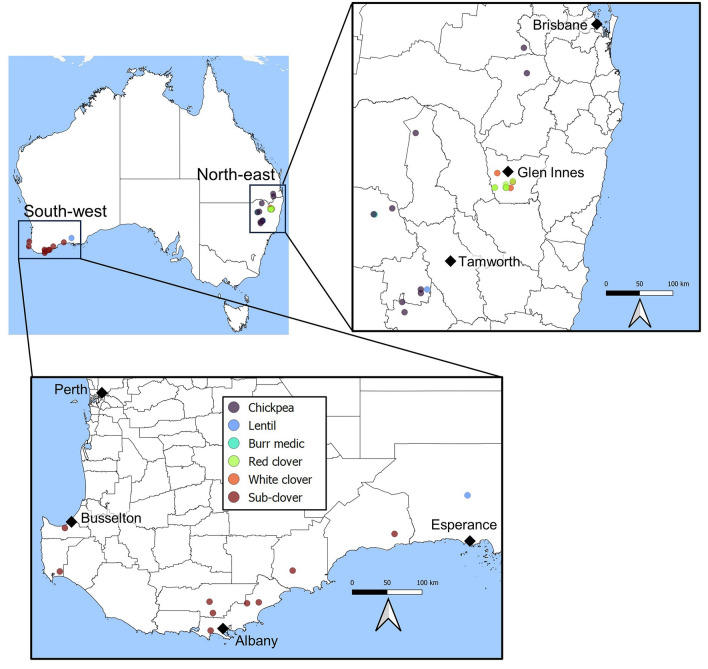
The 41 new SbDV isolates sequenced in this study were collected from two geographically distinct production regions of Australia; the south coast of south-west WA (hereafter referred to as the ‘south-west’) and a ~45,000-km2 area of northern NSW/southern QLD (hereafter referred to as the ‘north-east’) (Fig. 1, Table 1). From the south-west, 10 isolates were collected, including nine from subterranean clover growing in dairy pastures on the south coast and one from lentil growing in Grass Patch in the Esperance region. From the north-east, 31 isolates were collected. These consisted of 10 each from red clover and white clover from mixed pastures growing within 30 km of Glen Innes. The remaining nine from chickpea, one from lentil, and one from burr medic (Medicago polymorpha) were from sites spanning from Pilton, Queensland, in the north to Breeza, NSW, in the south.
Fig. 1.
Locations and hosts of soybean dwarf virus isolates sequenced from the south-coast of south-west Western Australia (south-west) and north-east New South Wales/south-east Queensland (north-east) regions of Australia
Table 1.
Details of soybean dwarf virus isolates sequenced in this study
| Accession number | Isolate | Source | Regiona | Location | Year collected | Predicted cladeb | Actual cladec |
|---|---|---|---|---|---|---|---|
| PP922787 | 5943 | Medicago polymorpha | NE | Edgeroi, NSW | 2013 | - | DP |
| PP922781 | 5433 | Cicer arietinum | NE | Croppa Creek, NSW | 2014 | - | DP |
| PP922786 | 5944 | C. arietinum | NE | Spring Ridge, NSW | 2015 | - | DP |
| PP922782 | 5434 | C. arietinum | NE | Breeza, NSW | 2015 | - | DP |
| PP922783 | 5436 | C. arietinum | NE | Edgeroi, NSW | 2015 | - | DU |
| PP922784 | 5481 | C. arietinum | NE | Colley Blue, NSW | 2018 | - | DP |
| PP922789 | L43 | Trifolium repens | NE | Lambs Valley, NSW | 2022 | Y | DP |
| PP922790 | L45 | T. repens | NE | Lambs Valley, NSW | 2022 | Y | DP |
| PP922791 | L46 | T. pratense | NE | Lambs Valley, NSW | 2022 | D | DP |
| PP922792 | L51 | T. pratense | NE | Glen Innes, NSW | 2022 | D | DP |
| PP922793 | L56 | T. pratense | NE | Matheson, NSW | 2022 | D | DP |
| PP922799 | L59 | T. pratense | NE | Matheson, NSW | 2022 | D | DP |
| PP922794 | L60 | T. pratense | NE | Shannon Vale, NSW | 2022 | D | DP |
| PP922795 | L69 | T. pratense | NE | Lambs Valley, NSW | 2022 | D | DP |
| PP922780 | L71 | T. pratense | NE | Lambs Valley, NSW | 2022 | D | DP |
| PP922796 | L72 | T. repens | NE | Lambs Valley, NSW | 2022 | Y | DP |
| PP922797 | L77 | T. pratense | NE | Lambs Valley, NSW | 2022 | D | DP |
| PP922798 | L87 | T. pratense | NE | Lambs Valley, NSW | 2022 | D | DP |
| PP922788 | L42 | T. pratense | NE | Lambs Valley, NSW | 2022 | D | DP |
| PP922785 | 5483 | C. arietinum | NE | Breeza, NSW | 2018 | - | DP |
| PP922803 | 5435 | Lens culinaris | NE | Breeza, NSW | 2015 | - | YP |
| PP922802 | 5432 | C. arietinum | NE | Edgeroi, NSW | 2013 | - | YP |
| PP922811 | L49 | T. repens | NE | Glen Innes, NSW | 2022 | Y | YP |
| PP922812 | L54 | T. repens | NE | Reddestone, NSW | 2022 | Y | YP |
| PP922813 | L61 | T. repens | NE | Shannon Vale, NSW | 2022 | Y | YP |
| PP922814 | L70 | T. repens | NE | Lambs Valley, NSW | 2022 | Y | YP |
| PP922815 | L76 | T. repens | NE | Lambs Valley, NSW | 2022 | Y | YP |
| PP922816 | L85 | T. repens | NE | Lambs Valley, NSW | 2022 | Y | YP |
| PP922817 | L86 | T. repens | NE | Lambs Valley, NSW | 2022 | Y | YP |
| PP922809 | 5945 | C. arietinum | NE | Pilton, QLD | 2013 | - | YP |
| PP922808 | 5946 | C. arietinum | NE | Warwick, QLD | 2013 | - | YP |
| PP922804 | 6091 | T. subterraneum | SW | Esperance, WA | 2017 | - | YP |
| PP922806 | 6694 | T. subterraneum | SW | Narrikup, WA | 2017 | - | YP |
| PP922807 | 6931 | T. subterraneum | SW | Gairdner, WA | 2017 | - | YP |
| PP922805 | 6692 | T. subterraneum | SW | Mt Barker, WA | 2017 | - | YP |
| PP922800 | BC2020 | L. culinaris | SW | Grass Patch, WA | 2019 | - | YP |
| PP922801 | 3342 | T. subterraneum | SW | Green Range, WA | 2019 | - | YP |
| PP922810 | KF20 | T. subterraneum | SW | Scott River, WA | 2020 | - | YP |
| PP922819 | WA8 | T. subterraneum | SW | Torbay, WA | 2018 | YP | YP |
| PP922820 | McG | T. subterraneum | SW | Busselton, WA | 2020 | - | YP |
| PP922818 | SS1 | T. subterraneum | SW | South Stirlings, WA | 2020 | - | YP |
aProduction regions of Australia: south coast of south-west Western Australia (SW) and north-east New South Wales/south-east Queensland (NE)
bBased on available biological data – Y or D based on host range indicators (T. repens, T. pratense, Phaseolus vulgaris, Lupinus albus) and P or S based on primary vector species (Acyrthosiphon pisum or Aulacorthum solani, respectively)
cBased on clade in whole-genome nt sequence tree (Y or D) and N-terminal region of the readthrough domain aa sequence tree (P or S). U – undetermined
RNA extraction and PCR confirmation
All RNA extractions were done on fresh or freeze-dried material using QIAshredder and RNeasy Mini Kits according to the manufacturer's instructions (QIAGEN, Germany). Two-step RT-PCR and Sanger sequencing were performed to confirm the presence of SbDV. Generic ‘Luteoviridae’ primers AS2 (5’- ATCACBTTCGGGCCGWSTYTWTCAGA-3’) and AS3 (5’- CACGCGTCIACCTATTTIGGRTTITG -3’) were used to amplify a region of ORF3 [1]. To obtain cDNA, reverse transcription was performed using an ImProm-II Reverse Transcription System with random primers (Promega, USA). The cDNA was used to perform PCR amplification using GoTaq DNA polymerase (Promega, USA) with the reaction consisting of an initial incubation at 95°C for 1 min followed by 30 cycles of 95°C for 15 s, 50°C for 20 s, and 72°C for 60 s and a final extension at 72°C for 10 min. The product was analysed by 1% agarose gel electrophoresis to confirm bands and then purified using a QIAquick PCR Purification Kit according to the manufacturer's instructions (QIAGEN, Germany). The purified product was then sent to the Australian Genome Research Facility (AGRF) for Sanger sequencing. The resulting sequences were confirmed to be SbDV using the BLAST tool in Geneious Prime 2022.0.1 (Biomatters, New Zealand).
RNA sequencing and genome sequence assembly
Total RNA of each isolate was sent to AGRF for plant ribosomal RNA depletion, library preparation, and barcoding before being sequenced on an Illumina NovaSeq instrument (Illumina, USA).
For each sample, reads were first trimmed using CLC Genomics Workbench (CLCGW) (formerly CLC Bio, Denmark, now QIAGEN, Germany) with the quality scores limit set to 0.01, the maximum number of ambiguities set to two, and removing any reads with <30 nucleotides (nt). Contigs were assembled using the de novo assembly function of CLCGW with automatic word size; automatic bubble size; minimum contig length, 500; mismatch cost, 2; insertion cost, 3; deletion cost, 3; length fraction, 0.5; and similarity fraction, 0.9. Contigs were sorted by length, and the longest was used as a query sequence for a BLAST search [2]. In addition, trimmed reads were imported into Geneious Prime 2022.0.1 and provided with a reference sequence obtained from the GenBank database (Table 2). Mapping was performed with a minimum overlap of 10%, a minimum overlap identity of 80%, "allow gaps" set to 10%, and fine-tuning set to iterate up to 10 times. The contig of interest from CLCGW and the consensus sequence from mapping in Geneious were used to create a consensus sequence in Geneious by alignment using Clustal W. ORFs were predicted and annotations were made using Geneious. Finalized sequences were submitted to GenBank (accession numbers PP922780-PP22820).
Table 2.
Summary of sequencing data from 41 new soybean dwarf virus isolates
| GenBank accession no. | Isolate ID | Number of reads | No. of reads after trimming | No. of contigs after assembly | Contig length (nt) | Reads mapped (de novo) | Average coverage (de novo) | Reference | Number of reads mapped to reference | Average coverage (mapped) | Final length (nt) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PP922804 | 6091 | 22,104,898 | 22,092,373 | 20,469 | 5,700 | 1,386,835 | 23,105 | NC003056 | 1,452,334 | 22,638 | 5,609 |
| PP922806 | 6694 | 20,628,914 | 20,613,554 | 20,115 | 5,926 | 207,565 | 3,408 | n/a | n/a | n/a | 5,609 |
| PP922807 | 6931 | 22,607,850 | 22,592,006 | 31,839 | 5,887 | 1,702,978 | 27,895 | n/a | n/a | n/a | 5,609 |
| PP922805 | 6692 | 19,395,142 | 19,383,697 | 21,324 | 5,878 | 1,314,610 | 21,560 | n/a | n/a | n/a | 5,609 |
| PP922800 | BC2020 | 53,522,120 | 53,522,098 | 25,478 | 5,831 | 1,173,236 | 19,004 | n/a | n/a | n/a | 5,604 |
| PP922801 | 3342 | 39,230,352 | 38,334,678 | 20,459 | 5,917 | 689,614 | 11,447 | n/a | n/a | n/a | 5,609 |
| PP922810 | KF20 | 41,097,054 | 40,365,759 | 27,674 | 3,748; 1,142 | 1,041,052; 548,224 | 27,295; 46,052 | LR584028 | 1,756,848 | 28,687 | 5,609 |
| PP922819 | WA8 | 57,384,178 | 55,951,527 | 30,772 | 5,950 | 552,795 | 9,146 | n/a | n/a | n/a | 5,609 |
| PP922820 | McG | 53,079,454 | 51,851,854 | 25,577 | 6,037 | 3,479,926 | 56,778 | n/a | n/a | n/a | 5,611 |
| PP922818 | SS1 | 48,673,758 | 45,577,214 | 22,218 | 5933 | 3,519,284 | 58,145 | n/a | n/a | n/a | 5,609 |
| PP922802 | 5432 | 62,076,358 | 60,037,094 | 32,357 | 5,108 | 2,608,915 | 50,176 | n/a | n/a | n/a | 5,609 |
| PP922787 | 5943 | 52,993,582 | 51,377,805 | 30,309 | 4,908; 1,096 | 3,401,863; 871,626 | 66,628; 74,596 | n/a | n/a | n/a | 5,459 |
| PP922809 | 5945 | 54,675,340 | 54,433,481 | 33,980 | 1,782; 1,202; 656; 737 | 1,928,006; 814,347; 146,719; 496,744 | 99,885; 65,075; 20,387; 58,227 | n/a | n/a | n/a | 5,697 |
| PP922808 | 5946 | 42,405,138 | 42,129,029 | 25,414 | 5,876 | 2,265,159 | 38,413 | n/a | n/a | n/a | 5,701 |
| PP922781 | 5433 | 46,109,438 | 45,908,319 | 22,653 | 5,190 | 5,680,097 | 108,557 | n/a | n/a | n/a | 5,459 |
| PP922786 | 5944 | 57,146,620 | 56,927,262 | 19,071 | 5,706 | 712,689 | 12,448 | n/a | n/a | n/a | 5,459 |
| PP922782 | 5434 | 47,162,646 | 46,922,166 | 23,475 | 5,756 | 5,187,565 | 89,779 | n/a | n/a | n/a | 5,459 |
| PP922803 | 5435 | 53,056,370 | 52,900,341 | 27,969 | 2,214; 5,950 | 95,844; 1,317,816 | 4,214; 22,001 | n/a | n/a | n/a | 5,609 |
| PP922783 | 5436 | 51,172,706 | 50,927,899 | 23,750 | 5,827 | 6,099,515 | 104,271 | n/a | n/a | n/a | 5,459 |
| PP922784 | 5481 | 71,168,032 | 70,928,034 | 19,809 | 5,749 | 48,487,240 | 840,894 | n/a | n/a | n/a | 5,459 |
| PP922785 | 5483 | 50,322,106 | 49,976,747 | 21,143 | 5,853 | 10,778,403 | 183,595 | n/a | n/a | n/a | 5,459 |
| PP922788 | L42 | 40,614,886 | 40,614,796 | 38,311 | 2966; 1723; 1091 | 624997; 308805; 117918 | 27301; 21971; 13950 | MF627965 | 1,245,669 | 29,738 | 5,459 |
| PP922789 | L43 | 34,250,078 | 34,249,989 | 27,449 | 1235; 4298 | 954; 2943 | 101; 93 | MF627965 | 4081 | 95 | 5,459 |
| PP922790 | L45 | 32,285,868 | 32,285,769 | 28,350 | 5,719 | 30,552 | 730 | n/a | n/a | n/a | 5,459 |
| PP922791 | L46 | 36,191,964 | 36,191,870 | 29,004 | 1415; 1388; 1094; 1163; 696 | 148405; 68970; 78667; 71214; 20253 | 14080; 6750; 9779; 8147; 3980 | MF627965 | 551,306 | 13,051 | 5,459 |
| PP922811 | L49 | 32,620,208 | 32,620,129 | 30,069 | 2814; 735; 550; 927 | 135428; 24610; 23075; 279 | 6234; 4284; 5060; 39 | LR584028 | 244,004 | 5,485 | 5,609 |
| PP922792 | L51 | 57,525,816 | 57,525,689 | 15,412 | 1271; 868; 1011; 1511; 715 | 18611; 3556; 12542; 24761; 10081 | 1819; 538; 1694; 2084; 1677 | MF627965 | 85,570 | 1,988 | 5,459 |
| PP922812 | L54 | 36,607,670 | 36,607,571 | 54,297 | 3896; 951; 1032; 618 | 18901; 856; 3798; 3646 | 636; 114; 482; 754 | LR584028 | 28,039 | 632 | 5,609 |
| PP922793 | L56 | 42,220,748 | 42,220,654 | 38,069 | 1827; 1119; 989; 772; 736; 764 | 153713; 86009; 23873; 22865; 7766; 42536 | 11449; 10175; 3207; 3663; 1332; 7555 | MF627965 | 443,643 | 10,573 | 5,459 |
| PP922799 | L59 | 34,097,278 | 34,097,190 | 29,816 | 1541; 687; 879; 664; 638 | 42035; 15556; 12163; 20023; 16201 | 3388; 3014; 1692; 3989; 3269 | MF627965 | 149,239 | 3,492 | 5,459 |
| PP922794 | L60 | 33,559,768 | 33,559,668 | 29,417 | 4445; 1078;1197 | 389872; 86205; 38284 | 11742; 10317; 3427 | MF627965 | 520,441 | 12,346 | 5,459 |
| PP922813 | L61 | 51,478,278 | 51,478,160 | 49,745 | 5,852 | 344,489 | 8,006 | NC003056 | 344,009 | 7,455 | 5,609 |
| PP922795 | L69 | 32,645,862 | 32,645,787 | 26,197 | 833; 845; 1083; 756; 546 | 23087; 47204; 78341; 53305; 28774 | 3680; 6762; 9650; 8625; 6739 | MF627965 | 321,165 | 21,526 | 5,459 |
| PP922814 | L70 | 42,457,582 | 42,457,486 | 33,471 | 1471; 1651; 892 | 218610; 164822; 85476 | 18993; 12947; 12790 | LR584028 | 657,403 | 15,227 | 5,609 |
| PP922780 | L71 | 37,889,610 | 37,889,513 | 27,161 | 1813; 686; 576; 620; 995; 571 | 143555; 34656; 39517; 6082; 3360; 4089 | 10244; 5222; 9180; 1119; 276; 602 | MF627965 | 404,881 | 9,414 | 5,456 |
| PP922796 | L72 | 64,673,360 | 54,673,190 | 49,915 | 1600; 980; 2579; 1506; 1491; 1173 | 115905; 27536; 268243; 98190; 36396; 1548 | 9601; 3708; 14223; 8841; 3188; 170 | MF627965 | 545,039 | 12,983 | 5,459 |
| PP922815 | L76 | 33,648,108 | 33,648,038 | 29,579 | 6,021 | 276,910 | 6,258 | n/a | n/a | n/a | 5,609 |
| PP922797 | L77 | 35,807,912 | 35,807,823 | 28,675 | 5,695 | 246,185 | 5,901 | n/a | n/a | n/a | 5,459 |
| PP922816 | L85 | 38,030,960 | 38,030,858 | 30,805 | 1729; 866; 932; 865; 701; 678 | 165881; 84164; 59107; 20979; 35343; 50424 | 12860; 12828; 7962; 3138; 6094; 9747 | LR584028 | 513,228 | 11,861 | 5,609 |
| PP922817 | L86 | 38,375,122 | 38,375,022 | 31,052 | 680; 840; 715; 1316; 1012; 699 | 62832; 40110; 10896; 68542; 40892; 12155 | 11677; 5778; 1821; 6840; 5238; 2120 | LR584028 | 291,276 | 6,936 | 5,609 |
| PP922798 | L87 | 28,236,728 | 28,236,652 | 21,438 | 2667; 853; 707; 675 | 101429; 11163; 2734; 18087 | 4844; 1686; 497; 3119 | MF627965 | 177,534 | 4,090 | 5,459 |
Phylogenetic analysis
All 50 available complete or near-complete genome sequences of SbDV, including three from Australia (Table 3), were downloaded from GenBank and aligned with the 41 new genome sequences from this study, using MAFFT [31]. The N-RTD sequence was extracted from the nucleotide sequence alignment and translated to an aa sequence alignment before analysis. Phylogenetic analysis was performed using the maximum-likelihood method and the HKY model with uniform rates for the nt alignment, and the maximum-likelihood method and the JTT matrix-based model for the aa alignment, both in MEGA X [34]. Pairwise nt % and aa % identity values were calculated in Geneious 2022.0.1, using the same alignments. Bean leafroll virus (accession number NC003369) was used as an outgroup for both trees.
Table 3.
Soybean dwarf virus sequences obtained from GenBank and used in phylogenetic analysis
| Accession number | Isolate | Host | Location | Year collected | Reference | Predicted cladea | Actual cladeb |
|---|---|---|---|---|---|---|---|
| AB038150 | M96-1 (DP) | Aphid | Japan | 2001 | [50] | DP | DP |
| MN412737 | Kreis_Stormarn_16 | Pisum sativum | Germany | 2016 | [16] | - | DP |
| MN412738 | Kreis_Stormarn_18 | P. sativum | Germany | 2018 | [16] | - | DP |
| MG600300 | SDV-HZ3 | Trifolium pratense | Czechia | 2015 | [35] | D | DP |
| MG600299 | SDV-HZ1 | T. pratense | Czechia | 2015 | [35] | D | DP |
| MF627965 | HS128 | Vigna angularis | Korea | 2016 | Unpublished | - | DP |
| OM953424 | HS | Glycine max | Korea | 2020 | [24] | - | DP |
| MT526793 | IA-2016 | G. max | USA | 2016 | [13] | - | DP |
| MT526794 | IA-2017 | G. max | USA | 2017 | [13] | - | DP |
| MT669395 | IA-2-2018 | G. max | USA | 2018 | [13] | - | DP |
| MT669394 | IA-1-2018 | G. max | USA | 2018 | [13] | - | DP |
| KJ786321 | C1IL2 | T. pratense | USA | 2009 | [52] | D | DP |
| DQ145545 | Wisc3 | G. max | USA | 2003 | [9] | D | DP |
| KJ786322 | W4 | G. max | USA | 2009 | [52] | - | DP |
| OK030799 | Market Weighton | P. sativum | UK | 2019 | [14] | - | DP |
| OR553429 | MD2-D | T. repens | USA | 1991 | [47] | DP | DR |
| OR553431 | MD3-D | Chenopodium spp. | USA | 1991 | [47] | DP | DP |
| OR553432 | MD7-D | G. max | USA | 1993 | [47] | DP | DP |
| OR553433 | MD8-D | Medicago lupulina | USA | 1988 | [47] | DP | DP |
| OR553434 | MD9-D | T. pratense | USA | 2005 | [47] | DP | DP |
| OR553435 | MD12-D | T. incarnatum | USA | 2006 | [47] | DP | DP |
| OR553439 | NY-D | T. pratense | USA | 1988 | [47] | DP | DP |
| OR553440 | PA-D | T. hybridum | USA | 1988 | [47] | DP | DP |
| OR553441 | SC-D | T. subterraneum | USA | 1991 | [47] | DP | DP |
| OR553442 | VA20-D | T. subterraneum | USA | 1990 | [47] | DP | DP |
| AB038149 | HS97-8 (DS) | G. max | Japan | 2001 | [50] | DS | DS |
| AB076038 | HS99-5 (DS) | G. max | Japan | 1999 | [51] | DS | DS |
| OR553424 | Hok2-D | G. max | Japan | 1981 | [47] | DS | DS |
| LR584030 | ESPCL2 | T. subterraneum | Esperance, Aus | 2013 | [32] | - | YP |
| LR584029 | ESPCL15-2 | T. subterraneum | Esperance, Aus | 2013 | [32] | - | YP |
| AB038148 | M94-1 (YP) | G. max | Japan | 2001 | [50] | YP | YP |
| MT543032 | JKI ID 23556 | T. repens | Germany | 2007 | [15] | YP | YP |
| MN412736 | Muenster_16 | P. sativum | Germany | 2016 | [16] | - | YP |
| JN674402 | MD6-Y | T. repens | USA | 2006 | [53] | YP | YP |
| LR584028 | NSWCP15-2 | Cicer arietinum | NSW, Aus | 2013 | [32] | - | YP |
| OK030752 | East Anglia | P. sativum | UK | 2007 | [14] | - | YP |
| OR553426 | SY-Y | Lens culinaris | Syria | 1994 | [47] | YP | YP |
| OR553427 | KY-Y | T. repens | USA | 1990 | [47] | YP | YP |
| OR553428 | MD1-Y | T. repens | USA | 1986 | [47] | YP | YP |
| OR553430 | MD2-Y | T. repens | USA | 1991 | [47] | YP | YP |
| OR553436 | MD13-Y | T. repens | USA | 2006 | [47] | YP | YP |
| OR553437 | MS-Y | T. subterraneum | USA | 1989 | [47] | YP | YP |
| OR553438 | NC-Y | T. repens | USA | 1990 | [47] | YP | YP |
| OR553443 | VA20-Y | T. subterraneum | USA | 1990 | [47] | YP | YP |
| OL472235 | MIR20SW | Pooled weeds | Slovenia | 2020 | [43] | - | YP |
| AB038147 | M93-1 (YS) | G. max | Japan | 2001 | [50] | YS | YS |
| L24049 | Tas-1 | Vicia faba | Tasmania, Aus | ~1980s | [42] | YS | YS |
| OR553423 | Hok1-Y | G. max | Japan | 1981 | [47] | YS | YS |
| OR553425 | NZ-Y | T. repens | New Zealand | 1986 | [47] | YS | YS |
| LC663963 | RG24 | G. max | Japan | 2017 | [18] | - | YS |
| NC003369c | Bean leafroll virus | V. faba | USA | - | [10] | - | - |
aBased on available relevant biological data – Y or D based on host range indicators (T. repens, T. pratense, Phaseolus vulgaris) and P or S based on vector species (Acyrthosiphon pisum or Aulacorthum solani)
bBased on clade in the whole-genome nt sequence tree (Y or D) and N-terminal region of the readthrough domain aa sequence tree (P or S), R -recombinant
cUsed as outgroup in phylogenetic trees
Results
High-throughput sequencing
Across all 41 samples, the total number of reads after trimming for each sample ranged from 19,383,142 to 70,928,034. The sequences were assembled and/or mapped to a reference sequence, and the final genome sequences obtained were 5,511 nt to 5,752 nt in length, with an average coverage from 39 times to 840,894 times (across complete and partial de novo-assembled segments). The data for each sample, including any references used for mapping are shown in Table 2. In total, 41 SbDV genome sequences were obtained, all of which can be considered ‘near-complete’, containing the entire coding region and much of the 5' and 3' untranslated regions.
Phylogenetic analysis of whole-genome nt sequences – D and Y clades
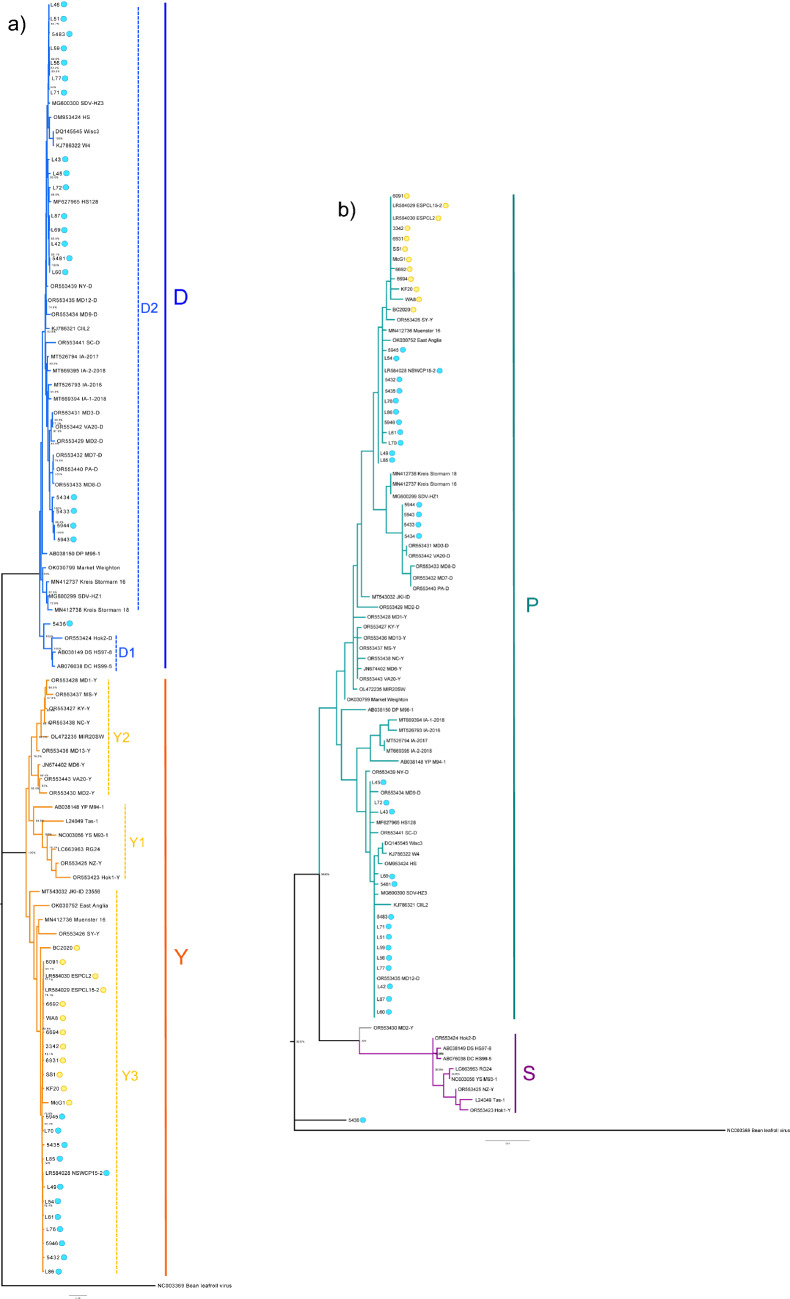
When analysing the nt sequence of the whole genome, SbDV isolates separated into distinct D and Y clades with 77-80% nt sequence identity between them (Fig. 2a). Australian isolates were represented in both D and Y clades. Among the available isolates, there was more diversity within the Y clade than within the D clade. However, among Australian isolates, Y clade isolates were slightly less diverse (23 of 24 isolates had 99 to 100% nt sequence identity, and one had 95 to 97% nt sequence identity) than D clade isolates (95 to 100% nt sequence identity) despite having a broader geographical distribution.
Fig. 2.
Mainland Australian isolates:  = north-east,
= north-east,  = south-west. Soybean dwarf virus phylogenetic tree of 92 whole-genome nucleotide sequences, including the reference sequence of bean leafroll virus used as an outgroup. The maximum-likelihood method and the Tamura-Nei model were used with 1000 bootstrap replicates. Annotations of D and Y subclades are as identified by Stone et al. [47]. (a) Phylogenetic tree of 92 partial amino acid sequences of the N-RTD region of soybean dwarf virus, including the reference sequence of bean leafroll virus as an outgroup. The maximum-likelihood method and the JTT matrix-based model were used with 1000 bootstrap replicates (b) Both trees shown here are the ones with the highest log likelihood. The percentage of trees in which the associated sequences clustered together is shown next to the branches.
= south-west. Soybean dwarf virus phylogenetic tree of 92 whole-genome nucleotide sequences, including the reference sequence of bean leafroll virus used as an outgroup. The maximum-likelihood method and the Tamura-Nei model were used with 1000 bootstrap replicates. Annotations of D and Y subclades are as identified by Stone et al. [47]. (a) Phylogenetic tree of 92 partial amino acid sequences of the N-RTD region of soybean dwarf virus, including the reference sequence of bean leafroll virus as an outgroup. The maximum-likelihood method and the JTT matrix-based model were used with 1000 bootstrap replicates (b) Both trees shown here are the ones with the highest log likelihood. The percentage of trees in which the associated sequences clustered together is shown next to the branches.
The analysis supported the D and Y subclades identified by Stone et al. [47]. All mainland-Australian YP clade isolates originating from subterranean clover in the south-west and chickpea, lentil, and white clover in the north-east were highly similar, clustering in the Y3 subclade with isolates from Germany, the United Kingdom, and Syria. The 10 south-west isolates formed a tight cluster in the Y3 subclade with >95% nt sequence identity. Subterranean-clover-infecting isolates collected in 2013 from the south-west and sequenced in a previous study [32] had 99-100% nt sequence identity to isolates originating from subterranean clover growing 300-700 km to the west from 2017 to 2020. In the north-east, all 11 Y clade isolates sequenced in this study and a chickpea-infecting isolate collected in 2013 in a previous study [32] had 99% nt sequence identity and also fell into the Y3 subclade. These grouped with the south-west Y clade isolates, mostly with 99% nt sequence identity, except for two south-west isolates: McG1 (97 to 98% nt sequence identity) from Busselton, with variation concentrated in ORF1, and BC2020 (96 to 97% nt sequence identity) from Grass Patch, with variation concentrated in the 3’ half of ORF5 (a variable region of the SbDV genome). These isolates had 95% nt sequence identity to each other. The only other Y clade isolate sequence from Australia was Tas-1, which fell in the Y1 subclade with isolates from New Zealand and Japan.
The 20 D clade isolates from the north-east had ~80% nt sequence identity to Y clade isolates from the same region, and sometimes from the same pasture sward (e.g., isolates L45 and L70). Among the D clade isolates, there was 95 to 100% identity, with 19 isolates falling into the D2 subclade with isolates from the eastern USA, Korea, the Czech Republic, Germany, the United Kingdom, and Japan. The most divergent isolate, 5436 from chickpea, fell outside the D1 and D2 subclades, with 96% nt sequence identity to both D1 and D2 subclade isolates. The D clade isolates infecting red clover swards in 2022 were 97 to 100% identical to all other chickpea-infecting isolates and the one medic-infecting isolate collected 200 to 300 km to the south and west between 2013 and 2018. None of the south-west isolates fell into the D clade.
Phylogenetic analysis of N-RTD aa sequences – P and S clades
When analysing the N-RTD aa sequence, 81 out of 91 sequences grouped in the P clade, eight grouped in the S clade, and two fell outside the two clades (5436 and MD2-Y) (Fig. 2b). Isolate Tas-1, originating from Tasmania and transmitted by Au. solani, fell into the S strain clade. The other 44 Australian isolates fell into the P strain clade, including all of those sequenced in this study. Isolate 5436, which fell outside the P and S strain clades, had 89-93% aa sequence identity to the P clade isolates, 83-87% aa sequence identity to the S clade isolates, and 89% aa sequence identity to the recombinant isolate MD2-Y. Although the aa sequence from isolate 5436 had many unique aa substitutions, it had P-type residues at 11 of the 12 positions (E97 being the exception) identified by Stone et al [47] as potential determinants of vector specificity.
Discussion
In this study, we conducted phylogenetic analysis of the near-complete genome nt sequences and the N-RTD aa sequences of 44 SbDV isolates from mixed-cropping regions in the south-west and north-east of mainland Australia (41 newly sequenced in this study) together with 46 isolates from nine other countries. At the whole-genome level, the isolates separated into D and Y clades. At the N-RTD level, most of the isolates separated into P and S clades. All of the south-west isolates and 11 of the 31 north-east isolates were in the Y clade, and the remaining 20 north-east isolates were in the D clade. Except for one isolate that fell outside the P and S clades, all south-west and north-east isolates were in the P group. Host range and/or vector species data available for 34 of 50 isolates obtained from GenBank and 21 of 41 isolates from this study supported the inferences from phylogenetic analysis, except for three D clade isolates sequenced in this study that originated from white clover in a mixed red and white clover sward in the north-east. These analyses suggest that the YP strain is predominant in the south-west and YP and DP strains are predominant in the northeast, suggesting that Ac. pisum, M. persicae, and possibly Ap. craccivora are the key SbDV vectors in these regions and thus targets for effective virus management. The Australian SbDV phylogeny is an important resource for future research and will facilitate the development of robust strain-specific diagnostic assays.
The genetic similarity of south-west isolates collected over the past decade suggest that the YP strain and its vectors are involved in the repeated epidemics of leaf reddening disease in subterranean clover in the south-west [44]. This inference is supported by phenotypic data for one of these isolates (WA-8), which was transmitted by Ac. pisum and M. persicae and able to infect white clover, common bean, and albus lupin, but not red clover [6], as well as the known prevalence of SbDV in white clover pastures in this region [37, 38]. In the north-east, both DP and YP are implicated in disease of chickpea [46] and probably other grain legumes. Y and D isolates collected from white and red clover swards had a high degree of nt sequence similarity to isolates in the same clade collected from grain legumes ~250 km to the north, south, and west from 2013 to 2018 (99% and 95-100% for Y and D clade isolates, respectively). This suggests an epidemiological link between grain and pasture legume production across the region – i.e., widespread SbDV infection in perennial pasture/weed species such as white and red clover could be providing a sustained reservoir of both SbDV and its vectors for spread into sensitive grains crops. Given the prevalence of P strain isolates, the risk of an epidemic in both regions analysed is likely to be determined by the population growth and movement of P strain vectors between pastures/weeds and crops, both with the potential to play the role of virus/vector source. This information will enable management strategies that target these potentially crucial aspects of SbDV.
The genetic and biological data together suggest that at least three of the four SbDV strains are present in Australia (YP, YS, and DP) but have differing geographical distributions. The high similarity between YP strain isolates collected in the south-west and north-east likely reflects recent and related incursions of this strain into these regions. Ac. pisum was first identified on mainland Australia in Victoria in 1980, and it had spread throughout NSW by 1982 and was being reported in WA by the late 1980s [5, 11]. SbDV infection of subterranean clover was reported in both regions as early as 1984, but the strain responsible could not be deduced [20]. At that time, both M. persicae and Ap. craccivora had been distributed across Australia for at least several decades [12] and thus could have also introduced SbDV YP or DP into these regions. No DP isolates were detected in the south-west, which could be explained by the absence of significant red clover cultivation in the region. No S clade isolates were found in the south-west or north-east. Au. solani was responsible for the first reported SbDV outbreaks on mainland Australia, in Victoria in the mid-1960s, and was also present in NSW and QLD by the mid-1960s [12]. Therefore, it is probable that S strains are present in NSW and QLD in SbDV-susceptible crops, which Au. solani frequently colonises. Au. solani has been present in the south-west region for at least several decades [5] but is uncommon in the host species studied here. The geographical barrier of the Nullarbor Plain likely also plays a major part in the apparently narrower genetic diversity of SbDV in the south-west. Future work should involve sequencing isolates collected from the south-east of mainland Australia (south NSW and Victoria), including any archived isolates available from the early outbreaks in subterranean clover, to get a more comprehensive picture of SbDV diversity in grain and pasture legumes grown in Australia. Furthermore, surveillance studies could provide information about the prevalence and diversity of SbDV in horticultural areas across Australia and any links that exist to isolates found in broadacre production.
Over 90% of SbDV isolates now available in the GenBank database are P clade isolates, including all isolates from the United Kingdom, mainland Europe, mainland Australia, the USA, and Syria, whilst just eight S strain isolate sequences are available, and they are limited to Japan (including all DS isolates sequenced), Tasmania, and New Zealand, in which they are reported to be most common [3, 27, 55]. This mainly reflects the fact that the largest sequencing studies have been done in regions where S strains are uncommon or absent – i.e., a study involving sequencing of a comparable number of isolates from soybean in Japan or vegetable legumes in New Zealand would be expected to change the P/S sequence proportion. However, it is also apparent that S strains have a smaller vector range [26] and, by extension, a smaller effective host range, which contributes to their absence in many of the regions studied.
Three north-east D clade isolates (L43, L45, and L72) were found in white clover growing in a mixed red and white clover sward, providing supporting evidence that white clover is not a strict Y strain indicator host [45]. Schneider et al. [45] also found that Y isolates can infect red clover plants when coinfected with a D strain isolate. However, in other cases, red clover was completely resistant to Y isolates [6, 28], and no Y isolates were found in red clover in this study. Although no mixed infection of strains was detected in our study, mixed pasture swards would facilitate mixed infections and provide an opportunity for SbDV recombinants with unique phenotypes to emerge. Furthermore, there is evidence that vector specificity is not always strict. Ashby et al. [4] reported that an S isolate from New Zealand could be transmitted with poor efficiency by Ac. pisum, and Schneider et al. [45] found that a mixed infection of a YP and a DP isolate was transmitted inefficiently by Au. solani. Of all of the isolates, isolate 5436 was the most diverse globally in the N-RTD and fell outside the P and S clades, but it resembled the P type at most of the important residues that are potential determinants of vector specificity [47]. It is plausible that this variation may influence this isolate’s transmissibility and vector species range. More-comprehensive host and vector range studies of Australian isolates, especially those involving recombinants, unique isolates, and mixed infections, would allow the inferences made in this study to be tested and broaden our understanding of SbDV biology and its genetic influences.
SbDV isolates also vary in their virulence, i.e., the severity of disease caused. Helms et al. [19] examined the virulence of three Au. solani-transmitted isolates (NSW-B, NSW-K, and Tas-1) on subterranean clover and found that NSW-K was significantly more virulent. However, sequence data are available only for Tas-1. Just one other sequenced Australian isolate (WA-8) has been phenotyped for virulence, and it caused severe disease in subterranean clover, chickpea, lentil, faba bean, and field pea [6]. Damsteegt [7] demonstrated that a DS isolate and a YS isolate differed in their transmissibility, symptomatology, and virulence across different hosts. Stone et al. [47] found that Y isolates that cause severe disease in soybean clustered strongly in a phylogenetic tree based on the movement protein (ORF4), indicating that it could be a determinant of virulence. Comparing the virulence of genetically different isolates on key hosts such as subterranean clover and grain legumes would help to identify any genomic determinants of this important trait.
This study used established relationships between phylogenetic clades and phenotypes to infer biological information from analysis of plant virus sequence data. In recent years, the warranted enthusiasm around new diagnostic and genome sequencing technologies has come at the cost of generating accompanying biological data [23]. Now that genome sequencing is an established tool in plant virology, a renewed focus on phenotyping genetic variants is likely to provide transformative meaning and value to the data generated by sequencing.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
We thank K. Foster, P. Sanford, J. van Leur, J. George, and the growers and consultants who collected SbDV-infected plant samples, J. Baulch and C. Wang at DPIRD, who assisted with preparing samples for sequencing, and H. Spafford for reviewing the late stage manuscript. This work was funded by DPIRD WA and Grains Research and Development project DAW2305-003RTX ‘Effective virus management in grains crops’.
Author contributions
B. Congdon conceptualised the study, processed and submitted the isolates for sequencing, and wrote the manuscript. M. Sharman provided many of the north-east isolates and edited the manuscript. M. Kehoe conducted all the bioinformatics, submitted the sequences to GenBank, and edited the manuscript.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Data availability
The datasets generated and/or analysed in the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Abraham A, Varrelmann M, Vetten H (2008) Molecular evidence for the occurrence of two new luteoviruses in cool season food legumes in Northeast Africa. Afr J Biotech 7:414–420. 10.5897/AJB07.717 [Google Scholar]
- 2.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410. 10.1016/S0022-2836(05)80360-2 [DOI] [PubMed] [Google Scholar]
- 3.Ashby JW, Teh PB, Close RC (1979) Symptomatology of subterranean clover red leaf virus and its incidence in some legume crops, weed hosts, and certain alate aphids in Canterbury, New Zealand. New Zeal J Agric Res 22:361–365. 10.1080/00288233.1979.10430760 [Google Scholar]
- 4.Ashby JW, Fletcher JD, Farrell JAK, Stufkens MR (1982) Observations on host preferences and epidemiology of aphid species associated with legume crops. New Zeal J Agric Res 25:267–272. 10.1080/00288233.1982.10420923 [Google Scholar]
- 5.Berlandier F (1997) Distribution of aphids (Hemiptera: Aphididae) in potato growing areas of Southwestern Australia. Aust J Entomol 36:365–375. 10.1111/j.1440-6055.1997.tb01487.x [Google Scholar]
- 6.Congdon BS, Baulch JR, Foster KJ (2023) Vector species, pasture legume host range, and impact on grain legumes of an Australian soybean dwarf virus isolate. Arch Virol 168:20. 10.1007/s00705-022-05664-8 [DOI] [PubMed] [Google Scholar]
- 7.Damsteegt VD (1990) Soybean dwarf virus: experimental host range, soybean germ plasm reactions, and assessment of potential threat to U.S. Soybean production. Plant Dis 74:992–995. 10.1094/PD-74-0992 [Google Scholar]
- 8.Damsteegt VD, Stone AL, Kuhlmann M, Gildow FE, Domier LL, Sherman DJ, Tian B, Schneider WL (2011) Acquisition and Transmissibility of U.S. Soybean dwarf virus Isolates by the Soybean Aphid, Aphisglycines. Plant Dis 95:945–950. 10.1094/PDIS-10-10-0726 [DOI] [PubMed] [Google Scholar]
- 9.Domier L, Thekke-Veetil T, Phibbs A, Barta A (2005) Complete nucleotide sequence of a Wisconsin soybean isolate of Soybean dwarf virus. Phytopathology 95:S25 [Google Scholar]
- 10.Domier LL, McCoppin NK, Larsen RC, D’Arcy CJ (2002) Nucleotide sequence shows that Bean leafroll virus has a Luteovirus-like genome organization. J Gen Virol 83:1791–1798. 10.1099/0022-1317-83-7-1791 [DOI] [PubMed] [Google Scholar]
- 11.Dominiak B, Walters P (1984) Establishment of Acyrthosiphon pisum (Harris)(Hemiptera: Aphididae) in New South Wales. Aust J Entomol 23:269–270. 10.1111/j.1440-6055.1984.tb01959.x [Google Scholar]
- 12.Eastop V (1966) A taxonomic study of Australian Aphidoidea (Homoptera). Aust J Zool 14:399–592. 10.1071/ZO9660399 [Google Scholar]
- 13.Elmore MG, Groves CL, Hajimorad MR, Stewart TP, Gaskill MA, Wise KA, Sikora E, Kleczewski NM, Smith DL, Mueller DS, Whitham SA (2022) Detection and discovery of plant viruses in soybean by metagenomic sequencing. Virol J 19:149. 10.1186/s12985-022-01872-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fowkes AR, McGreig S, Pufal H, Duffy S, Howard B, Adams IP, Macarthur R, Weekes R, Fox A (2021) Integrating High throughput Sequencing into Survey Design Reveals Turnip Yellows Virus and Soybean Dwarf Virus in Pea (Pisum Sativum) in the United Kingdom. Viruses 13:2530. 10.3390/v13122530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaafar Y, Ziebell H (2020) Complete Genome Sequence of a Soybean Dwarf Virus Isolate from White Clover in Germany. Microbiol Resour Announce 9:28. 10.1128/mra.00637-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaafar YZA, Herz K, Hartrick J, Fletcher J, Blouin AG, MacDiarmid R, Ziebell H (2020) Investigating the Pea Virome in Germany—Old Friends and New Players in the Field(s). Front Microbiol 11:583242. 10.3389/fmicb.2020.583242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gildow FE, Damsteegt VD, Stone AL, Smith OP, Gray SM (2000) Virus-vector cell interactions regulating transmission specificity of Soybean Dwarf Luteoviruses. J Phytopathol 148:333–342. 10.1046/j.1439-0434.2000.00518.x [Google Scholar]
- 18.Hagiwara-Komoda Y (2022) An efficient mechanical inoculation technique for soybean dwarf virus reveals that the viral readthrough domain is inessential for the systemic infection of host plants. J Gen Plant Pathol 88:197–202. 10.1007/s10327-022-01057-6 [Google Scholar]
- 19.Helms K, Waterhouse PM, Carver M (1983) Aulacorthum (neomyzus) circumflexum, a vector of subterranean clover red leaf virus. Austral Plant Pathol 12:66–67. 10.1071/APP9830066 [Google Scholar]
- 20.Helms K, Muller W, Waterhouse P (1993) National survey of viruses in pastures of subterranean clover. I. Incidence of four viruses assessed by ELISA. Aust J Agirc Res 44:1837–1862. 10.1071/AR9931837 [Google Scholar]
- 21.Honda K, Kanematsu S, Mikoshiba Y (1999) Dwarfing strain of soybean dwarf luteovirus transmitted by Nearctaphis bakeri and Acyrthosiphon pisum. Ann Phytopathol Soc Jpn 65:387 [Google Scholar]
- 22.Honda K (2001) Aphids and their transmission of viruses on soybeans in Japan. Agrochem Jpn 79:2–7 [Google Scholar]
- 23.Hou W, Li S, Massart S (2020) Is there a “biological desert” with the discovery of new plant viruses? A retrospective analysis for new fruit tree viruses. Front Microbiol 11:592816. 10.3389/fmicb.2020.592816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jo Y, Choi H, Lee BC, Hong J-S, Cho WK (2022) Complete genome sequence of soybean dwarf virus infecting soybean (Glycine max L.). Korean J Microbiol 58:91–95. 10.7845/kjm.2022.2020 [Google Scholar]
- 25.Johnstone GR (1978) Diseases of broad bean (Vicia faba L. major) and green pea (Pisum sativum L.) in Tasmania caused by subterranean clover red leaf virus. Aust J Agric Res 29:1003–1010. 10.1071/AR9781003 [Google Scholar]
- 26.Johnstone GR, Patten DS (1981) Sub-clover red leaf and sub-clover stunt viruses are not transmitted by lucerne and pea aphids. Austral Plant Pathol 10:65–66. 10.1071/APP9810065 [Google Scholar]
- 27.Johnstone GR, Rapley P (1981) Control of subterranean clover red leaf virus in broad bean crops with aphicides. Ann Appl Biol 99:135–141. 10.1111/j.1744-7348.1981.tb05140.x [Google Scholar]
- 28.Johnstone GR, Ashby JW, Gibbs AJ, Duffus JE, Thottappilly G, Fletcher JD (1984) The host ranges, classification and identification of eight persistent aphid-transmitted viruses causing diseases in legumes. Neth J Plant Pathol 90:225–245. 10.1007/BF01976381 [Google Scholar]
- 29.Johnstone GR, McLean GD (1987) Virus diseases of subterranean clover. Ann appl Biol 110:421–440. 10.1111/j.1744-7348.1987.tb03274.x [Google Scholar]
- 30.Jones RAC (2012) Virus diseases of annual pasture legumes: incidences, losses, epidemiology, and management. Crop Pasture Sci 63:399–418. 10.1071/CP12117 [Google Scholar]
- 31.Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30:772–780. 10.1093/molbev/mst010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kehoe MA, Coutts BA (2019) Turnip yellows virus and Soybean dwarf virus in Western Australia. Austral Plant Pathol 48:323–329. 10.1007/s13313-019-00632-4 [Google Scholar]
- 33.Kellock A (1971) Red-leaf virus - a newly recognized virus disease of subterranean clover (Trifolium subterraneum L.). Aust J Agric Res 22:615–624. 10.1071/AR9710615 [Google Scholar]
- 34.Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549. 10.1093/molbev/msy096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lenz O, Sarkisová T, Koloniuk I, Fránová J, Přibylová J, Špak J (2018) Red clover-associated luteovirus – a newly classifiable member of the genus Luteovirus with an enamo-like P5 protein. Arch Virol 163:3439–3442. 10.1007/s00705-018-3997-1 [DOI] [PubMed] [Google Scholar]
- 36.Makkouk KM, Kumari SG (2001) Reduction of incidence of three persistently transmitted aphid-borne viruses affecting legume crops by seed-treatment with the insecticide imidacloprid (Gaucho®). Crop Prot 20:433–437. 10.1016/S0261-2194(00)00169-1 [Google Scholar]
- 37.McKirdy S, Jones R (1995) Occurrence of alfalfa mosaic and subterranean clover red leaf viruses in legume pastures in Western Australia. Aust J Agric Res 46:763–774. 10.1071/AR9950763 [Google Scholar]
- 38.McKirdy SJ, Jones RAC (1997) Further studies on the incidence of virus infection in white clover pastures. Aust J Agric Res 48:31–38. 10.1071/A96040 [Google Scholar]
- 39.Meat and Livestock Australia (2020) The economic significance of Australia’s red meat and livestock industry. Available at: https://www.mla.com.au/prices-markets/market-news/2020/the-economic-significance-of-australias-red-meat-and-livestock-industry/. Accessed 10 Feb 2022
- 40.Mikoshiba Y, Fujisawa I, Honda K (1991) A new strain of soybean dwarf virus transmitted by Acyrthosiphon pisum in Japan. Ann Phytopathol Soc Jpn 57:448 [Google Scholar]
- 41.Norton MR, Johnstone GR (1998) Occurrence of alfalfa mosaic, clover yellow vein, subterranean clover red leaf, and white clover mosaic viruses in white clover throughout Australia. Aust J Agric Res 49:723–728. 10.1071/A97114 [Google Scholar]
- 42.Rathjen JP, Karageorgos LE, Habili N, Waterhouse PM, Symons RH (1994) Soybean dwarf luteovirus contains the third variant genome type in the luteovirus group. Virol 198:671–679. 10.1006/viro.1994.1079 [DOI] [PubMed] [Google Scholar]
- 43.Rivarez MPS, Pecman A, Bačnik K, Maksimović O, Vučurović A, Seljak G, Mehle N, Gutiérrez-Aguirre I, Ravnikar M, Kutnjak D (2023) In-depth study of tomato and weed viromes reveals undiscovered plant virus diversity in an agroecosystem. Microbiome 11:60. 10.1186/s40168-023-01500-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanford P, Congdon BS, Foster KJ (2021) Identifying the cause of recent outbreaks of subterranean clover red leaf syndrome in Western Australia. Australian Grassland Association Virtual Symposium 2021, 10-31 March 2021
- 45.Schneider WL, Damsteegt VD, Stone AL, Kuhlmann M, Bunyard BA, Sherman DJ, Graves MV, Smythers G, Smith OP, Hatziloukas E (2011) Molecular analysis of soybean dwarf virus isolates in the eastern United States confirms the presence of both D and Y strains and provides evidence of mixed infections and recombination. Virol 412:46–54. 10.1016/j.virol.2011.01.001 [DOI] [PubMed] [Google Scholar]
- 46.Sharman M, Moore K, van Leur JAG, Aftab M, Verrell A (2014) Impact and management of viral diseases in chickpeas. Grains Research and Development Updates 2014. Available at: https://grdc.com.au/resources-and-publications/grdc-update-papers/tab-content/grdc-update-papers/2014/03/impact-and-management-of-viral-diseases-in-chickpeas. Accessed 30 June 2024
- 47.Stone AL, Damsteegt VD, Smith OP, Stewart LR (2024) Global phylogenetic analysis of soybean dwarf virus isolates and their associations with aphid vectors and severe disease in soybeans. Virol 591:109984. 10.1016/j.virol.2024.109984 [DOI] [PubMed] [Google Scholar]
- 48.Tamada T (1970) Aphid transmission and host range of soybean dwarf virus. Ann Phytopath Soc Japan 36:266–274. 10.3186/jjphytopath.36.266 [Google Scholar]
- 49.Tamada T (1975) Studies on the soybean dwarf disease. Rep Hokkaido Prefect Agric Exp Stn 25:1–144 [Google Scholar]
- 50.Terauchi H, Kanematsu S, Honda K, Mikoshiba Y, Ishiguro K, Hidaka S (2001) Comparison of complete nucleotide sequences of genomic RNAs of four Soybean dwarf virus strains that differ in their vector specificity and symptom production. Arch Virol 146:1885–1898. 10.1007/s007050170040 [DOI] [PubMed] [Google Scholar]
- 51.Terauchi H, Honda K-I, Yamagishi N, Kanematsu S, Ishiguro K, Hidaka S (2003) The N-terminal region of the readthrough domain is closely related to aphid vector specificity of Soybean dwarf virus. Phytopathology 93:1560–1564. 10.1094/PHYTO.2003.93.12.1560 [DOI] [PubMed] [Google Scholar]
- 52.Thekke-Veetil T, McCoppin N, Domier L (2017) Strain-specific association of soybean dwarf virus small subgenomic RNA with virus particles. Virus Res 242:100–105. 10.1016/j.virusres.2017.09.003 [DOI] [PubMed] [Google Scholar]
- 53.Tian B, Gildow FE, Stone AL, Sherman DJ, Damsteegt VD, Schneider WL (2017) Host adaptation of soybean dwarf virus following serial passages on pea (Pisum sativum) and soybean (Glycine max). Viruses. 10.3390/v9060155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walker P, Siddell S, Lefkowitz E, Mushegian A, Adriaenssens E, Alfenas-Zerbini P, Davison A, Dempsey D, Dutilh B, Garcia ML, Harrach B, Harrison R, Hendrickson R, Junglen S, Knowles N, Krupovic M, Kuhn J, Lambert A, Lobocka M, Zerbini F (2021) Changes to virus taxonomy and to the International Code of Virus Classification and Nomenclature ratified by the International Committee on Taxonomy of Viruses (2021). Arch Virol 166:1–16. 10.1007/s00705-021-05156-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilson J, Close RC (1973) Subterranean clover red leaf virus and other legume viruses in Canterbury. New Zeal J Agric Res 16:305–311. 10.1080/00288233.1973.10421108 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analysed in the current study are available from the corresponding author on reasonable request.