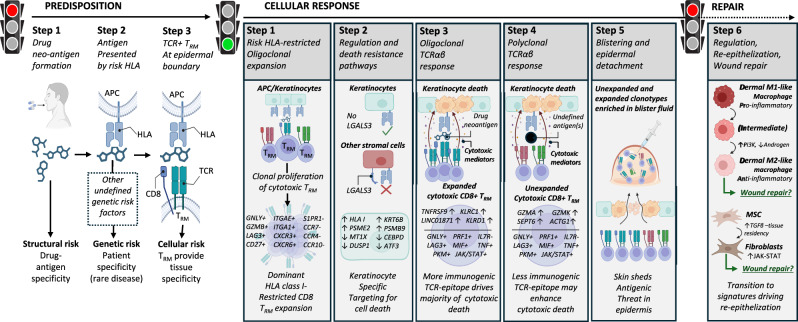
Fig. 6. A common pathogenic population of locally proliferating cytotoxic CD8+ LAG3+ TRM T cells with private expanded and unexpanded TCRαβ clonotypes drives keratinocyte-specific cell death across patients with diverse HLA-restricted drug-induced SJS/TEN.
Predisposition: To develop SJS/TEN, the patient must be exposed to the drug neo-antigen (Step 1: structural risk) and carry a particular HLA risk allele for that drug. The risk HLA allele is necessary but not sufficient for the onset of SJS/TEN, and other currently undefined genetic risk factors may also contribute to disease predisposition (Step 2. Complete genetic risk). The patient must also have cytotoxic CD8+ TRM T cells in the skin and mucous membranes with TCR specificity for the HLA risk-restricted drug-neo-antigen. These cells do not recirculate (Step 3. HLA- and drug neoantigen-specific tissue-resident CD8+ T cells provide the tissue specificity of SJS/TEN). While the drug, HLA, and TRM clonotype may be different between individual patients, the resulting cellular response is shared. Cellular response: Drug-neoantigen is presented by keratinocytes and potentially other stromal cells in the skin by the HLA class I risk allele. HLA class I risk-restricted CD8+ LAG3+ TRM T cells with specificity for the risk HLA-drug neoantigen complex proliferate locally in the skin. In response to local proliferation and inflammation, keratinocytes regulate pathways associated with regulation and death resistance, enabling keratinocyte-specific targeting by cytotoxic LAG3+ CD8+ TRM T cells. The HLA class I-restricted and clonally-expanded cytotoxic CD8+ TRM population initiates keratinocyte death. An unknown antigen or antigens then triggers a similar population of unexpanded cytotoxic CD8+ TRM T cells. We hypothesize that this is either a range of alternate drug neoantigens or a broader array of new antigens produced by the pathological process, and these unexpanded but cytotoxic clonotypes may enhance cytotoxic keratinocyte death. Keratinocyte death leads to epidermal separation and the formation of a sub-epidermal cleft and blister. Both expanded and unexpanded cytotoxic CD8+ TRM T cells enter the cleft and become concentrated in the blister fluid, with eventual separation of the epidermis from the dermis. Repair: Pro-inflammatory M1 and intermediate-like macrophages increase PI3K and androgen signaling towards anti-inflammatory populations associated with re-epithelization and repair. MSC increase TGFβ signaling and differentiate into fibroblasts which express JAK-STAT pathways and are associated with wound repair. TGFβ is also known to promote T cell tissue residency. Created in BioRender. Phillips, E. (2024) BioRender.com/n10c928. APC, antigen-presenting cell; HLA, human leukocyte antigen; TCR, T cell receptor; TRM, tissue-resident memory, MSC, mesenchymal stromal cell.