Abstract
Gastric Cancer (GC) is the fifth most common cancer worldwide. Early stages of GC began being detected, giving rise to a new concern, Quality of Life. This study aimed to systematically assess the effects of different GC reconstruction techniques on postoperative type 2 diabetes mellitus (T2DM), hypertension (HBP), and body mass index (BMI) reduction rate and to provide an overview of recent research on oncometabolic surgery (OS). We performed a systematic review and meta-analysis by searching three databases according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. We performed a meta-analysis of risk ratios and mean differences to estimate the impact of duodenal bypass, Roux-en-Y reconstruction, and residual stomach on T2DM, HBP, and BMI reduction rate. Heterogeneity was assessed using the I2 statistics. At the end of the follow-up, the duodenal bypass group compared to Billroth I had a significantly higher postoperative remission of T2DM and HBP, with a relative risk (RR) of 1.43 (95% confidence interval (95% CI) [1.27; 1.62]) and 1.3 (95% CI [1.00; 1.69]), respectively. Compared with the Billroth II group, Roux-en-Y reconstruction had significantly greater T2DM remission after gastrectomy (RR = 1.19; 95% CI [1.08; 1.31]), while HBP showed no significant differences. Regarding the improvement of HBP, total gastrectomy was significantly superior to subtotal gastrectomy (95% CI [1.01; 2.64]). A trend towards Roux-en-Y Esophagojejunostomy as the best option for T2DM remission was observed (95% CI [0.98; 2.77]; p = 0.06). Gastrectomy with Roux-en-Y reconstruction appears to be the most effective treatment for T2DM remission. Further research is needed to assess the impact of OS on metabolic diseases.
Keywords: Oncometabolic surgery, Gastric cancer, Gastrectomy, Gastric bypass, Metabolic diseases, Metabolic syndrome
Subject terms: Cancer, Endocrinology, Gastroenterology, Health care, Medical research, Oncology
Introduction
Gastric Cancer (GC) is the fifth most common cancer worldwide and the fourth leading cause of cancer-related deaths1. The standard treatment for gastric carcinoma remains radical resection with total or subtotal gastrectomy2,3.
The implementation of national screening programs made it possible to identify patients in the early stages of the disease, leading to a decline in GC mortality rates4,5. With the rise in life expectancy of patients with GC, a new health factor has emerged: Quality of Life. This topic is particularly important for patients with concurrent chronic diseases such as type 2 diabetes mellitus (T2DM), hypertension (HBP), and dyslipidaemia6.
Traditionally, bariatric surgery has been used to treat morbid obesity. However, it rapidly became clear its effectiveness in treating chronic comorbidities in obese patients, such as T2DM, dyslipidaemia, and HBP. These conclusions gave rise to the concept of Metabolic Surgery7.
Recently, based on the similarities between GC and bariatric surgery (including gastric resection and foregut bypass), surgeons have hypothesized that GC surgery could have beneficial effects on glycemic control. These findings led to the emergence of “Oncometabolic Surgery”8, a dual-purpose surgery with the potential to treat oncologic conditions while simultaneously improving patients’ quality of life by ameliorating chronic metabolic diseases with debilitating consequences9.
The primary purpose of this systematic review and meta-analysis was to provide an overview of recent research findings on oncometabolic surgery (OS). The second goal was to evaluate whether GC surgery helps oncologic patients improve their metabolic status. Additionally, we sought to assess the most suitable reconstructive technique for achieving the best metabolic profile.
Methods
This systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines10.
Eligibility criteria
We included all observational studies and randomized controlled trials analyzing patients that underwent gastrectomy for GC with concurrent metabolic diseases (T2DM, HBP, visceral fat, and dyslipidaemia).
We included studies assessing the outcomes of complete or partial remission of T2DM and HBP, body mass index (BMI) reduction rate, visceral and subcutaneous fat reduction rate, changes in total cholesterol (TC), low-density lipoprotein (LDL), high-density lipoprotein (HDL), and triglycerides (TG).
We excluded studies with small sample sizes (i.e., less than ten patients) or those that did not specify the reconstruction method used. All the reports identified as animal trials, editorials, correspondence, video reports, reviews, or meta-analyses were excluded. We did not apply a language restriction.
Information sources and search strategy
We searched three databases (PubMed, Scopus, and Web of Science) through December 2023. This search was conducted using the following Query: ((Bariatric Surgery) AND (Gastric Cancer)) OR (Oncometabolic). This Search Query was reinforced by a Grey Literature.
Study selection and data collection process
The study selection was divided into two phases. In the screening phase, after duplicate removal, each study was independently assessed and selected by title and abstract reading by two reviewers (MC, CO). In the second phase, study selection was based on full-text reading. Any disagreements were resolved by a third researcher (HSS).
Regarding the data collection process, both reviewers (MC, CO) extracted data from the selected articles separately using a predesigned data extraction sheet developed according to the Cochrane Handbook11. For each study, the following information was extracted: authors’ identification, year of publication, country, study design, number of participants, follow-up, participants’ inclusion and exclusion criteria, general characteristics of the participants (age, gender, mean duration T2DM, mean BMI, mean weight, mean hemoglobin A1c (HbA1c), mean fasting plasma glucose (FPG), mean systolic and diastolic blood pressure, TC, LDL, HDL, TG, and visceral and subcutaneous fat area), the metabolic diseases evaluated by the study (T2DM, HBP, adiposity, and dyslipidaemia), GC type and stage, neoadjuvant or adjuvant therapy, type of surgery (total or partial gastrectomy), reconstruction method (Roux-en-Y, Billroth I, and Billroth II) and outcome measures.
Quality assessment
The quality of the articles was independently analyzed by two reviewers (MC, CO) using the National Institutes of Health quality assessment criteria for observational studies12, and the Cochrane risk of bias tool for randomized control trials13.
The first tool consists of a form with 14 yes-or-no questions (related to research question, study population, exposure, outcome, blinding, follow-up, and statistical analysis), obtaining a final quality rating (ranging from good to poor) to classify the study according to its potential risk of bias (Supplementary Table 1).
The second tool includes stratification of the risk of selection, performance, detection, attrition, reporting bias, and other sources biases and the classification of the studies as high, low, or unclear (Supplementary Fig. 1).
Quantitative synthesis of results
We performed meta-analyses of relative risks for dichotomous variables (complete and partial remission of T2DM and HBP), and mean differences for continuous variables (BMI reduction rate). BMI reduction rate is a measure that is calculated as [(preoperative BMI − postoperative BMI) ÷ (preoperative BMI)] × 100.
For each outcome, the pooled meta-analytical measure, and the respective 95% confidence interval (95% CI) were obtained. We performed a random-effects meta-analysis using the restricted maximum likelihood approach.
Heterogeneity was assessed using Cochran’s Q test p-value and I2 statistic. Heterogeneity was considered high for p-values of the Cochran’s Q test lower than 0.10 or I2 above 50%. To explore potential sources of heterogeneity, we performed leave-one-out sensitivity analyses and, for analyses including more than ten primary studies, meta-regression analyses, testing follow-up time, preoperative BMI, and BMI reduction rate as moderator variables.
A p-value lower than 0.05 was considered statistically significant. This meta- analysis was performed using meta package of software R.
Results
Study selection
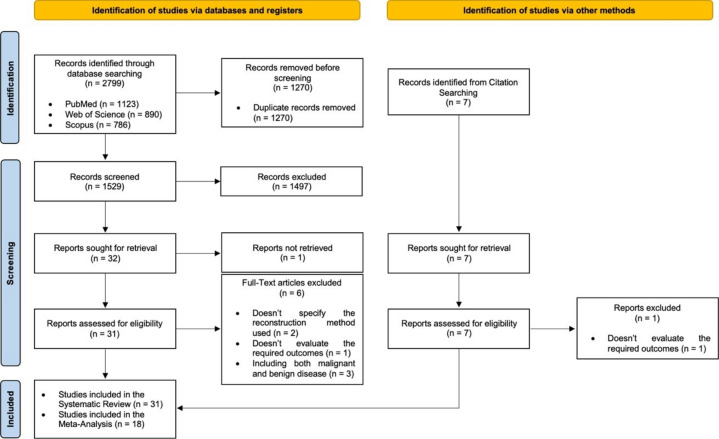
During the initial search, 2799 records were identified. After excluding duplicates, 1529 articles remained. After the screening phase, 31 articles were fully read, of which 25 were included. Through Grey Literature, 7 articles arose, and 6 of them were included. The quantitative synthesis comprised 18 papers out of the 31 included in the qualitative synthesis (Fig. 1)10.
Fig. 1.
Flow diagram of study selection.
Study characteristics
A summary of the study’s characteristics is presented in Table 1. The 18 articles included in the meta-analysis comprised of 3453 patients. Of the 31 eligible studies, 18 were retrospective studies8,14–30, 11 were prospective studies31–41, and 2 were randomized controlled trials42,43. The articles were published between 2012 and 2022 and were conducted in Korea, Japan, Taiwan, China, and Brazil. The minimum follow-up time of the included studies was 3 months (Zhang et al. 37), with five studies having a follow-up period lower than 12 months, fourteen studies between 12 and 24 months and twelve studies higher than 24 months.
Table 1.
General characteristics of the included studies.
| Study, year | Country | Study design | Nº patients | Mean Age (years) | Male (%) | Mean follow- up (months) | GC ataging | Neoadjuvant or adjuvant therapy | Interventio n type | Mean BMI (kg/m2) | BMI reductio n ratio (%) | Mean HbA1c (%) | Mean FPG (mg/dL) | Mean duration of T2DM (months) | Mean SBP (mmHg) | Mean DBP (mmHg) | Mean TC (mg/dL) | Mean LDL (mg/dL) | Mean HD L (mg/dL) | Mean TG (mg/dL) | Mean SFA (cm2) | Mean VFA (cm2) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lee et al.24 | Korea | RS | 220 | 64.5 | 77.8 | 12 | I, II, III | Adjuvant Therapy | BI, BII, RYGJ, RYEJ | 23.9 | 7,82 | – | 150.9 | – | – | – | – | – | – | – | – | – |
| Kang et al.25 | Korea | RS | 75 | 67 | 72 | 35 | – | Adjuvant Therapy | BI, BII, RYEJ | 23.8 | 8.03 | 7.4 | – | – | – | – | 176.77 | – | – | – | – | – |
| Kim et al.36 | Korea | RS | 403 | 63.8 | 76.2 | 33.7 | – | – | RYEJ, BI, BII | 24.7 | 9.8 | – | – | – | – | – | – | – | – | – | – | – |
| Zhang et al.37 | China | PS | 21 | 55.3 | 83.33 | 3 | I, II, III | No | BII | 22.4 | – | 7 | 140.0 | – | – | – | – | – | – | – | – | |
| Hayashi et al.35 | Brazil | PS | 164 | 68.3 | 57.2 | 36 | – | – | RYEJ, RYGJ | 25.85 | 8.5 | – | 116.25 | – | – | – | 179.65 | – | – | – | – | – |
| An et al.34 | Korea | PS | 64 | 62.7 | 67.2 | 12 | I | No | BI, BII, RYEJ | 24.7 | 10.22 | 7.2 | 149.63 | 6.6 | – | – | – | 102.6 | 43.17 | 138.13 | – | – |
| Kim et al.40 | Korea | PS | 15 | 62.1 | 66.67 | 12 | I, II, III | Neoadjuvant and Adjuvant Therapy | LRYGJ, LRYEJ | < 30 | 13.89 | – | – | 136.9 | – | – | – | – | – | – | – | – |
| Tanaka et al.30 | Japan | RS | 152 | 62.8 | 69.74 | 6 | I, II, III | Adjuvant Therapy | BI, RYGJ | 22.6 | 9.36 | – | – | – | – | – | – | – | – | – | 118.15 | 77.9 |
| Wei et al.29 | China | RS | 67 | 65.7 | 70 | 57.4 | I, II, III, IV | Adjuvant Therapy | BII, RYEJ | – | 14.53 | – | – | – | – | – | – | – | – | – | – | – |
| Wang et al.15 | Taiwan | RS | 69 | 70.3 | 76.8 | 67.8 | – | – | RYGJ, RYEJ, BI, BII | 24.8 | 15.7 | – | 149.2 | – | – | – | – | – | – | – | – | – |
| Xiong et al.19 | China | RS | 54 | 52.2 | 68.52 | 6 | I, II, III, IV | – | LRYGJ, LRYEJ | – | – | 10.6 | 102.6 | 105.6 | – | – | – | – | – | – | – | – |
| Tanaka et al.42 | Japan | RCT | 221 | 64.1 | 67.9 | 12 | – | Adjuvant Therapy | BI, RYGJ | 22.55 | 9.22 | – | – | – | – | – | – | – | – | – | 121.5 | 88.25 |
| Kwon et al.28 | Korea | RS | 49 | 65.2 | 82.3 | 24 | I, II, III | Adjuvant Therapy | BI and BII | 25.07 | 7.58 | 7.3 | 145 | – | 120 | 75 | 162 | – | – | – | – | – |
| Zhu23 | China | RS | 292 | 64.9 | 69.5 | 24 | – | No | BI, BII, RYEJ | 22.4 | 10 | – | 102.6 | 97.2 | – | – | – | – | – | – | – | – |
| Liu32 | China | PS | 93 | 59 | 50.54 | 12 | – | – | BI, RYGJ, RYEJ | 29.31 | – | 9.27 | 184.86 | – | – | – | – | – | – | – | – | – |
| Pak20 | Korea | RS | 90 | 65.4 | 70 | 24 | I, II, III | – | BI, BII, RYEJ | 24.8 | 11 | 7.1 | 151.0 | 25.6 | 119 | – | – | – | – | – | – | – |
| Kim et al.36 | Korea | PS | 30 | 63.9 | 46.7 | 12 | I, II, III, IV | Adjuvant Therapy | LRYGJ | 26.8 | 15.25 | 7.6 | 144.7 | 100.8 | – | – | – | – | – | – | – | – |
| Ho et al.21 | Taiwan | RS | 579 | 57 | 67.7 | 47.1 | – | – | RYEJ | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Choi43 | Korea | RCT | 40 | 62.6 | 65 | 12 | I, II, III | No | RYGJ, BI | 25.65 | – | 7.4 | 135.15 | – | – | – | – | – | – | – | – | – |
| Guner et al.22 | Korea | RS | 238 | 62.9 | 75 | 24 | I, II | No | BI, BII | 24.3 | – | 7.2 | 137 | 96 | – | – | – | – | – | – | – | – |
| Lee et al.8 | Korea | RS | 42 | 68.7 | 68.6 | 60 | I, II, III | – | BI, BII, RYEJ | 25.1 | – | 6.9 | 139.6 | 152.4 | – | – | – | – | – | – | – | – |
| Min Jeong Park41 | Korea | PS | 52 | 65 | 73.08 | 12 | I, II, III | Adjuvant Therapy | BI, BII, RYGJ, RYEJ | 24.94 | – | – | – | – | – | – | – | – | – | 144.70 | 128.18 | |
| Lin et al26 | China | RS | 455 | 69.4 | 56.76 | 99 | I | No | BII, RYGJ | 21.6 | – | – | – | 100 | 123 | 74 | 163 | – | 54 | 81 | – | – |
| Kim et al.38 | Korea | PS | 66 | 62 | 65.3 | 12 | Ia, Ib | No | BI, BII, RYGJ, RYEJ | 25.5 | – | – | – | – | 125 | 82 | 178 | 111 | 45 | 110 | – | – |
| Park et a.31 | Korea | PS | 40 | 60 | 80 | 12 | I | Adjuvant Therapy | LRYGJ, RYGJ | 29.6 | 9.63 | 5.8 | – | – | 136 | 84.25 | – | – | – | – | – | – |
| Kim et al.27 | Korea | RS | 1305 | 58.55 | 65.4 | 12 | Ia, Ib, IIa | No | BI, BII, RYGJ | 23.63 | – | – | – | – | – | – | 182 | – | – | – | – | – |
| Kim et al.16 | Korea | RS | 226 | 63.05 | 77 | 12 | I | No | LRYGJ, BII | 25.59 | 11 | 7.25 | 149.55 | – | – | – | – | – | – | – | – | – |
| Peng et al.18 | China | RS | 143 | 65.1 | 63.6 | 6 | – | – | BI, BII, RYEJ, RYGJ | 23.4 | 12.82 | – | – | – | – | – | – | – | – | – | – | – |
| Park et al.33 | Korea | PS | 20 | 60.1 | 70 | 6 | I, II | No | BI, LBII, LRYGJ | 25.8 | 8.91 | 7.8 | 121.4 | 36 | – | – | – | – | – | – | – | – |
| Lee et al..39 | Korea | PS | 41 | 62.4 | 80.5 | 12 | Ia, Ib | No | BI, BII, RYGJ, RYEJ | 24.5 | 6.45 | 7.3 | 132.5 | 81.6 | – | – | – | – | – | – | – | – |
| Choi et al.14 | Korea | RS | 48 | 60.73 | 83.1 | 12 | I, II | No | LRYGJ, LRYEJ | 23.77 | 11.34 | 7.45 | 122.21 | – | – | – | – | – | – | – | – | – |
GC gastric cancer, BMI body mass index, HbA1c hemoglobin A1c, FPG fasting plasma glucose, T2DM type 2 diabetes mellitus, SBP systolic blood pressure, DBP diastolic blood pressure, TC total cholesterol, LDL low density lipoprotein, HDL high density lipoprotein, TG triglycerides, SFA subcutaneous fat area, VFA visceral fat area, RS retrospective study, PS prospective study, BI Billroth I, BII Billroth II, RYGJ Roux-en-Y Gastrojejunostomy, RYEJ Roux-en-Y Esophagojejunostomy, LRYGJ Long-limb Roux-en-Y Gastrojejunostomy, LRYTG Long-limb Roux-en-Y Esophagojejunostomy.
Two types of gastrectomy (subtotal and total) and three different reconstruction methods were studied: Billroth I (BI), Billroth II (BII), and Roux-en-Y (RY; conventional and long-limb).
Although the definition of complete remission and improvement of T2DM and HBP demonstrated small differences between studies, all of them used the reduction or absence of medication as complete or partial remission criteria.
Risk of bias of individual studies
Supplementary table and Fig. 1 display the findings of the risk of bias assessments of the included studies.
Regarding observational studies, none of the studies justified their sample size, nor was the participants' level of exposure hidden from the outcome assessors. Concerning the eighth and tenth parameters related to the level and assessment of exposure in observational studies, we considered them not applicable to all articles. Most studies had a low risk of bias for the evaluated parameters. Twenty-three (79.31%) articles were classified as good, and six (20.69%) as fair.
Five studies (17.24%) with a follow-up duration of < 12 months did not have a sufficient timeframe to identify potential associations between exposure and outcomes, as their follow-up duration was judged inadequate to properly assess the outcomes.
The two randomized controlled trials included in the qualitative synthesis were considered to have an overall low risk of bias. Only one parameter, the random sequence generation, in Tanaka et al.42, was recognized as having high risk of selection bias.
Comparison of the duodenal bypass reconstruction techniques (Roux-en- Y and Billroth II) with Billroth I
Type 2 diabetes mellitus
The frequency of patients who achieved diabetic remission at the end of follow- up period was evaluated in 13 studies8,15,17,20,22–25,28,33,34,39,41.
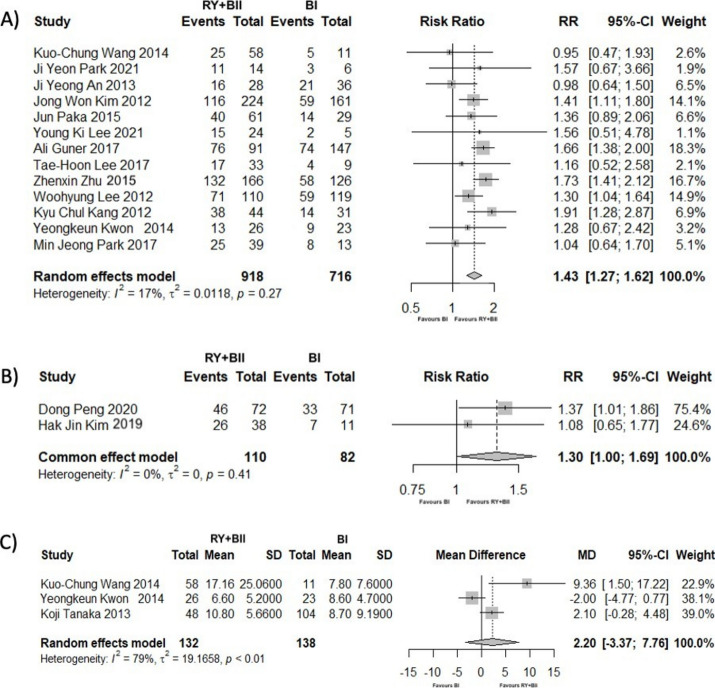
Patients who underwent gastrectomy with duodenal bypass reconstruction had 1.43 times more probability of T2DM improvement or remission compared to the BI reconstruction (RR = 1.43; 95% CI [1.27; 1.62]; p < 0.001). The heterogeneity between studies was moderate but not significant (I2 = 16.90%; p = 0.27) (Fig. 2A).
Fig. 2.
(A) Forest Plot showing the outcomes of complete or partial remission of T2DM after gastrectomy, comparing duodenal bypass to BI reconstruction, at the end of the follow-up period. (B) Forest Plot showing the outcomes of complete or partial remission of HBP after gastrectomy, comparing duodenal bypass to BI reconstruction, at the end of the follow-up period. (C) Forest Plot showing the outcomes of BMI reduction rate after gastrectomy, comparing duodenal bypass to BI reconstruction, at the end of the follow-up period.
Heterogeneity ceased to be observed using leave-one-out sensitivity analyses by omitting An et al.34 (Supplementary Table 2). To further explore the potential sources of heterogeneity, a meta-regression analysis was performed. None of the variables analyzed (follow-up time, preoperative BMI, and BMI reduction rate) were identified as heterogeneity moderators (Supplementary Table 3).
Hypertension
Two studies18,38 including 192 patients examined partial or complete HBP remission after duodenal bypass or BI reconstruction.
After gastrectomy, patients who received RY or BII reconstruction had a 1.3 higher likelihood of improving or having their HBP resolved (RR = 1.30; 95% CI [1.00; 1.69]; p = 0.048), with no heterogeneity being detected (I2 = 0%; p = 0.41) (Fig. 2B).
BMI reduction rate
Three studies15,28,30 (270 patients) compared surgical techniques regarding their BMI reduction rate after gastrectomy at the end of follow-up. There were no significant differences between BI and duodenal bypass reconstruction techniques in this parameter (MD = 2.20; 95% CI [− 3.37; 7.76]; p = 0.44). In addition, there was a high degree of heterogeneity within studies (I2 = 79.20%; p < 0.01) (Fig. 2C).
Comparison of the two duodenal bypass procedures, Roux-en-Y and Billroth II
Type 2 diabetes mellitus
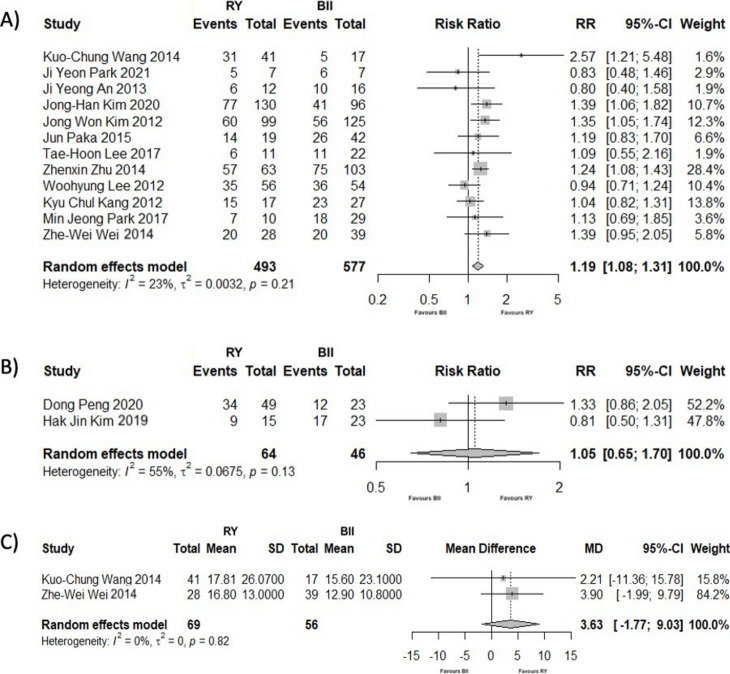
Twelve studies8,15–17,20,23–25,29,33,34,41 assessed the frequency of patients who had T2DM remission or improvement at the end of the follow-up period. The patients to whom gastrectomy with RY was performed had a 1.19 times greater chance of partial or complete remission compared to those with BII reconstruction (RR = 1.19; 95% CI [1.08; 1.31]; p < 0.001). The degree of heterogeneity was moderate, although not statistically significant (I2 = 23.40%; p = 0.21) (Fig. 3A).
Fig. 3.
(A) Forest Plot showing the outcomes of complete or partial remission of T2DM after gastrectomy, comparing RY to BII reconstruction, at the end of the follow-up period. (B) Forest Plot showing the outcomes of complete or partial remission of HBP after gastrectomy, comparing RY to BII reconstruction, at the end of the follow-up period. (C) Forest Plot showing the outcomes of BMI reduction rate after gastrectomy, comparing RY to BII reconstruction, at the end of the follow-up period.
Using leave-one-out sensitivity analyses, the heterogeneity decreased significantly (I2 = 3.60%) by omitting Wang et al.15 (Supplementary Table 4). None of the variables analyzed in meta-regression (follow-up time and preoperative BMI) were identified as heterogeneity moderators (Supplementary Table 5).
Hypertension
Two studies18,38 (110 patients) analyzed HBP remission or improvement at the end of the follow-up period. There were no significant differences between the RY and BII groups (RR = 1.05; 95% CI [0.65; 1.70]; p = 0.84), with heterogeneity being substantial (I2 = 55.40%; p = 0.13) (Fig. 3B).
BMI reduction rate
At the end of the follow-up period, the BMI reduction rates from two studies15,29 were compared (125 patients). No significant differences were observed between RY and BII reconstruction techniques (MD = 3.63; 95% CI [− 1.77; 9.03]; p = 0.19; I2=0%) (Fig. 3C).
Comparing Roux-en-Y between total gastrectomy and subtotal gastrectomy
Type 2 diabetes mellitus
To determine the impact of the residual stomach on T2DM remission, we compared RY reconstruction between total and subtotal gastrectomy, Roux-en-Y Esophagojejunostomy (RYEJ) and Roux-en-Y Gastrojejunostomy (RYGJ).
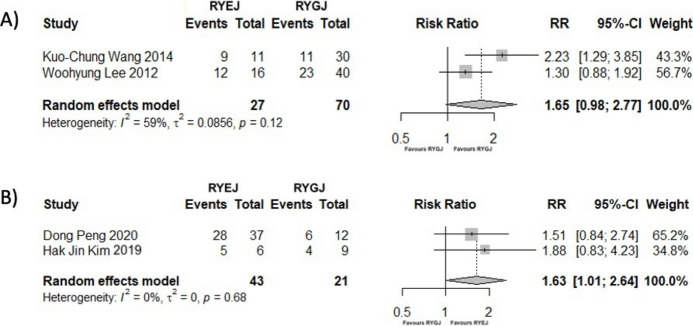
After total or subtotal gastrectomy with RY reconstruction, two studies15,24 (97 patients) evaluated complete or partial remission of T2DM. Figure 4A shows a trend favouring total gastrectomy, although no significant difference was observed (RR = 1.65; 95% CI [0.98; 2.77]; p = 0.06; I2 = 59.40%).
Fig. 4.
(A) Forest Plot showing the outcomes of complete or partial remission of T2DM, comparing RYEJ to RYGJ, at the end of the follow-up period. (B) Forest Plot showing the outcomes of complete or partial remission of HBP, comparing RYEJ to RYGJ, at the end of the follow-up period.
Hypertension
We examined Roux-en-Y reconstruction between total and subtotal gastrectomy to explore the impact of residual stomach on remission of HBP.
Two studies18,38 (64 patients) assessed the improvement or remission of HBP who underwent RYEJ or RYGJ. After RYEJ, patients had a 1.63 higher probability of improving their HBP (RR = 1.63; 95% CI [1.01; 2.64]; p = 0.046), with no heterogeneity detected (I2 = 0%; p = 0.68) (Fig. 4B).
Discussion
Summary of evidence
The mechanism underlying GC surgery as a metabolic surgery shares many similarities with bariatric Roux-en-Y Gastric Bypass (RYGB). Both rely on reduction of gastric volume and anatomical reconstruction of the stomach22. BI reconstruction preserves gastric tract continuity. In contrast, in reconstruction techniques that involve duodenal bypass, BII or RY, food bypasses the duodenum and proximal jejunum, reaching the distal ileum earlier. These two reconstruction techniques resemble bariatric surgery more than BI14.
Bariatric and Oncometabolic Surgery share many concepts, yet they also exhibit significant differences. One major drawback is their substantially different target population, candidate patients for OS tend to be older, frailer, and lighter9. Consequently, in contrast to obese individuals receiving bariatric surgery, inferior outcomes are to be anticipated in oncological patients undergoing gastrectomy. Patients with GC are less likely to be obese. Therefore, the effect of weight loss is smaller than what is expected in bariatric patients. Another disparity is their age: oncological patients are generally older. Ageing exponentially increases surgical morbidity and mortality, leading to higher and more severe preoperative comorbidities and postoperative risks44. Lastly, in conventional GC surgery, the bypass length is usually shorter than in bariatric RYGB, leading to a weaker enteric effect14.
Therefore, GC surgical techniques can be adjusted, thoroughly evaluated, and tailored to optimize therapeutic benefits without sacrificing oncological safety45 for a particular and cautiously selected patient group. OS involves adapting and choosing the best surgical method available for optimal oncologic and metabolic control.
To the best of our knowledge, this is the first meta-analysis exploring the impact of duodenal bypass (RY or BII), residual stomach, and the various GC reconstruction techniques on postoperative T2DM, HBP, and BMI reduction rate in patients with GC.
Duodenal Bypass demonstrated a significantly higher efficiency in the improvement of T2DM compared to BI reconstruction. For BII, the remission rate ranged from 13 to 83.5%, and for RY, it ranged from 30.7 to 90.5%. This wide variability may be attributed to distinct patient characteristics, different follow-up periods, lack of technical standardization, different lengths of the biliopancreatic and alimentary limbs, or the fact that almost all included studies, the primary goal was the resection of the tumor rather than the improvement of glycemic control.
The mechanism underlying T2DM remission after gastrectomy is similar to that in bariatric surgery. Two theories have been developed to explain this phenomenon: Foregut and Hindgut Hypotheses46. According to the Foregut Hypothesis, bypass of the duodenum alters levels of postprandial hormones, improving glucose control by enhancing insulin release and sensitivity. On the other hand, the Hindgut Hypothesis suggests that early contact of nutrients with the distal small intestine induces an antidiabetic effect by increasing GLP-1, which enhances insulin release and reduces postprandial hyperglycemia by slowing gastric emptying. Lastly, we must highlight the essential role of gut hormones in remission, not only in T2DM but also in HBP, dyslipidaemia, and cardiovascular events. Studies have demonstrated that, independent of weight loss, proximal intestinal bypass promotes T2DM improvement via incretins/anti-incretins, gut hormones, alteration in the gut microbiome, or altered bile acid signaling28,47,48.
According to several studies, the most significant independent predictors were the duration and severity of T2DM8,14,15,20–22,25,28,34,36,40,41 (preoperative diabetes duration, insulinogenic index, and HbA1c level) and weight loss8,17,20,22,34,36,40 (preoperative BMI, and BMI reduction rate). Several previous bariatric and gastrectomy studies have reported pancreatic β cell function as having an important role in T2DM remission49,50. Patients with shorter T2DM duration have greater β cell activity15. Therefore glycemic control would be more effective in GC patients with a shorter diabetes diagnosis time, not requiring insulin therapy, and with lower preoperative HbA1c levels8,20,21,25,28,36,41. Weight loss, higher preoperative BMI, and higher BMI reduction rate, even in nonobese patients, were also important factors in the improvement of glucose metabolism8,17,20,22,36,38.
Hypertension is a modifiable risk factor that is intimately linked to cardiovascular and cerebrovascular diseases and can affect the quality of life of cancer survivors38. Three articles18,31,38 from this qualitative synthesis evaluated the influence of gastrectomy and its different reconstruction methods on blood pressure control in patients with a BMI< 30 kg/m2. Remission and improvement of HBP after gastrectomy ranged from 53.1 to 67.4%. Two studies18,38 included in this quantitative synthesis showed significant superiority of duodenal bypass reconstruction over BI. However, no discernible difference was established between RY and BII. A statistically significant difference was observed in the extent of gastrectomy, demonstrating the superiority of RY Esophagojejunostomy. These findings were consistent with those in Peng et al., that reported that total gastrectomy was associated with a greater remission rate of HBP, and that no noticeable superiority was seen among the three reconstructive techniques for subtotal gastrectomy18. Both studies acknowledge that additional factors such as lifestyle modifications may play an important role.
The effect of GC surgery on metabolic control has been evaluated in several studies involving oncologic patients with BMI< 30 kg/m2. In fact, most have shown that T2DM, HBP, and dyslipidaemia improve following gastrectomy in non-obese patients23,25,28,31,38,41. However, although it has been proven that weight loss plays a crucial role in the metabolic status of patients with GC, suggesting that patients with a lower BMI are less likely to benefit from metabolic surgery, it is not the only contributing factor, nor is it statistically significant on its own20. Weight loss and the effects of duodenal bypass appear to work synergistically to improve metabolic control after gastrectomy, even in non-obese patients22. This suggests that while weight loss plays a role, other mechanisms, also significantly contribute to metabolic outcomes.
Cerebrovascular, cardiac, and respiratory diseases are common causes of death in patients with early GC52. One of the main findings of Lee et al. was that patients who underwent gastrectomy for early GC had a similar overall mortality as the general Korean population, but a significantly lower cardiovascular mortality53. Multiple studies included in this systematic review showed a significant lowering of TC, TG, and LDL levels and a rise of HDL after gastrectomy25–28,31,34,38.
Lin et al.26 states that GC patients who had undergone subtotal gastrectomy (BII and RY reconstruction) had significantly lower TC and TG levels, BMI, decreased waist circumferences, and occurrence of T2DM and Metabolic Syndrome. Lee et al. reinforced the correlation between weight loss and patients’ lipid profiles. After gastrectomy, a significant reduction in body weight and visceral fat might improve lipid metabolism and prevent atherosclerotic changes, leading to a reduction in cardiovascular mortality53.
One retrospective study and one randomized controlled trial (373 patients)30,42 evaluated the influence of RY and BI on visceral fat reduction after gastrectomy. Visceral fat accumulation has been identified as an underlying cause of metabolic syndrome54. These two studies showed that both duodenal bypass and gastrectomy promoted significant visceral fat loss.
Long-limb RY reconstruction has recently emerged as an option in GC surgery. Studies have suggested that increasing the length of biliopancreatic and alimentary limbs leads to better metabolic outcomes after gastrectomy43. Currently, no consensus on the optimal limb length has been established. Long-limb RY reconstruction typically refers to a modification of the standard procedure where the lengths of the anastomosis limbs exceed the traditional measurements. In conventional RY reconstruction surgery for gastric cancer, the length of the alimentary limb ranges from 40 to 50 cm, and the biliopancreatic limb is generally about 30–40 cm in length9.
Seven studies (433 patients)14,16,19,31,33,36,40 investigated the effect of long-limb RY reconstruction on T2DM improvement, ranging from 50.8 to 85%. These studies showed that a longer modification of RY resulted in better outcomes for T2DM, HBP, and dyslipidaemia. Kim et al. reported a statistically significant superiority of long-limb RY over the conventional BII in a multicenter retrospective cohort of 226 patients for up to 1 year after surgery16. Choi et al. found that the results of long-limb Roux-en-Y gastrojejunostomy (LRYGJ) in oncologic, diabetic, nonobese patients were similar to that of metabolic surgery in obese diabetic patients14. A prospective study of 40 patients compared long-limb RY with conventional RY for T2DM and HBP. It disclosed that the complication rate of OS group did not differ from the conventional group. Although no significant difference was observed between the two methods, the results showed higher remission rates for both T2DM (LRYGJ 77.8% and RYGJ 50%) and HBP (LRYGJ 68.8% and RYGJ 41.2%). Lastly, it reported an improvement in dyslipidaemia in 61.5% of patients in the OS group. However, this study had a small sample size, and its primary endpoint was nutritional safety not OS efficacy31.
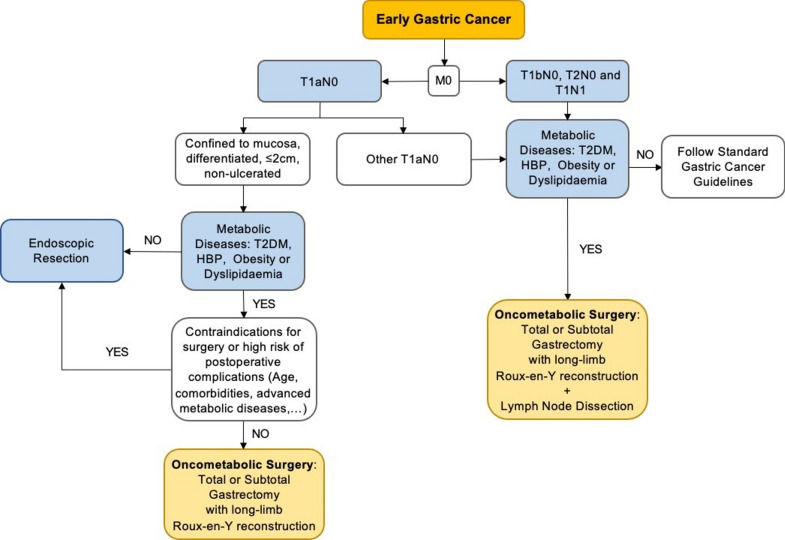
Who would benefit from OS? Patients with longer life expectancies, with localized early-stage GC, who would require ongoing care for their metabolic comorbidities: younger oncologic patients with shorter duration of T2DM, HBP, and dyslipidaemia, especially those who cannot adequately control their comorbidities by optimal lifestyle modification or pharmacotherapy. Although numerous studies have shown excellent outcomes in individuals with normal BMI, this surgery would be particularly favorable for patients with obesity. Figure 5 shows a potential treatment algorithm for patients with early GC and concomitant metabolic diseases, based on the ESMO Clinical Practice Guidelines55.
Fig. 5.
Potential treatment algorithm for early gastric cancer patients with metabolic diseases.
(Adapted from Kim et al.33 and ESMO Clinical Practice Guidelines55.
Limitations
Most included studies were based in Asia, specifically Korea, China, Japan, and Taiwan, except for Hayashi et al.35, which was from Brazil. The characteristics of Asian patients are distinct from those observed in other regions. There is a higher percentage of GC and less obesity in East Asia. This might be due to several risk factors, such as dietary patterns and the high prevalence of Helicobacter pylori infection56.
Second, most studies employed different criteria for remission and improvement of T2DM. Nevertheless, all studies used one common criterion: reduction or cessation of medication as indicators of partial or complete remission, respectively. In regard to T2DM, the majority of these studies adhered to the American Diabetes Association (ADA) criteria for defining complete and partial remission. According to the ADA, complete remission is characterized by a fasting blood glucose (FBS) level of less than 100 mg/dL and an HbA1c level below 6.0%, maintained for 1 year without the use of antidiabetic medication. Partial remission, on the other hand, is defined as a fasting blood glucose level between 100 and 125 mg/dL and an HbA1c level below 6.5%, which was maintained for 1 year in the absence of antidiabetic medication8,14,16,20,24,29,33,43. For HBP, both studies used reduction or cessation of medication as the criteria for determining partial or complete remission18,38.
Another limitation was the inability to analyze other key outcomes such as dyslipidaemia, visceral fat reduction, and other metabolic diseases. Data on such outcomes were not presented in the primary studies.
The influence of neoadjuvant and adjuvant therapy on glycemia, hypertension, and dyslipidemia in these patients was not deeply explored in the included studies, it is important to note that only one study briefly addressed the impact of adjuvant therapy. Lee et al. states that adjuvant chemotherapy in advanced stages could potentially influence glucose management. The most prevalent side effects are diminished appetite, nausea, vomiting, and growth hormone deficiency post-chemotherapy, which can impact insulin secretion24. Further research is needed to explore the influence of neoadjuvant and adjuvant therapies on glycemia, HBP, and dyslipidemia in gastric cancer patients. This area of study is crucial to better understand the comprehensive impact of these therapies on metabolic parameters and to optimize treatment outcomes.
Patients with GC and concurrent HBP after gastrectomy have rarely been reported. Further investigation is required on this subject as well as studies with larger sample sizes and longer follow-up times.
Most of the included studies reported weight reduction primarily using BMI reduction rates, which are commonly used in gastric cancer surgery, rather than the standard metrics used in bariatric surgery, such as %TWL, %EWL, or %EBMIL.
Further research on the efficiency of long-limb RY reconstruction with larger sample sizes and long-term outcomes is needed to compare its results with those of traditional BII and RY gastrectomy. In addition, a standard biliopancreatic limb length should be established to obtain optimal results.
All the outcomes analyzed in this meta-analysis were evaluated at the end of the follow-up period. Such periods ranged between 6 and 67.8 months. Three studies18,30,33 had a follow-up of 6 months, and four studies8,15,17,25 had follow- ups over 24 months. At both extremes, we observed significant improvements in the metabolic status. To evaluate whether this variation influenced heterogeneity, a meta- regression analysis was performed, which showed that this variable was not a heterogeneity moderator.
Lastly, some of our results demonstrated low to high heterogeneity. This heterogeneity might be due to disparity in patient characteristics, lack of standardization of biliopancreatic and alimentary limb lengths, and outcomes not being the primary goal of the study.
Conclusion
Our systematic review and meta-analysis demonstrated that both conventional and modified gastrectomy were effective in the improvement of metabolic diseases. However, RY reconstruction after gastrectomy is the most effective method for altering GC patients’ metabolic status.
The concept of OS could change the surgical oncology paradigm. Its future lies in surgeons actively selecting patients with specific characteristics (T2DM, obesity, HBP, dyslipidaemia, GC, and younger age) and modifying the traditional GC operation based on the principles of metabolic and bariatric surgery.
Future perspectives
Research on OS has mainly focused on T2DM rather than cardiovascular diseases or other metabolic comorbidities such as HBP, dyslipidaemia, obesity, and non-alcoholic fatty liver disease.
Further research with longer follow-up periods is needed to reflect the impact of OS on survival rates, quality of life and the safety of this procedure.
OS provides a glimmer for the prospective widening of the criteria for metabolic and bariatric surgery in the treatment of T2DM in non-obese, non- oncologic patients.
In future GC guidelines, OS should be considered as an option for patients with obesity or metabolic diseases.
Supplementary Information
Acknowledgements
First and foremost, we would like to thank Dr José Maximino Costa because, without his assistance and support, the progress and completion of this dissertation would not have been possible. The authors would like to acknowledge all the Obesity Integrated Responsibility Unit (CRI-O) group members.
Author contributions
Study concepts: M.P.C., H.S.S. Study design: M.P.C., H.S.S., B.S.—Pinto Data acquisition: M.P.C., Carolina Rodrigues Oliveira Quality control of data and algorithms: M.P.C., C.O., H.-S.S., B.S.-P. Data analysis and interpretation: M.P.C., C.O., H.-S.S., B.S.-P. Statistical analysis: M.P.C., B.S.-P. Manuscript preparation: M.P.C. Manuscript editing: M.P.C., C.O., H.-S.S., B.S.-P. Manuscript review: M.P.C., C.O., H.-S.S, B.S.-P., F.A.-C., R.B., E.B., S.C.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
Competing interests
The authors declare no competing interests.
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
For this type of study, formal consent is not required.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Maria Pinho Costa and Hugo Santos-Sousa.
These authors jointly supervised: Silvestre Carneiro and Bernardo Sousa-Pinto.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-72456-2.
References
- 1.Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.71(3), 209–249 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Ajani, J. A. et al. Gastric cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Canc. Netw.20(2), 167–192 (2022). [DOI] [PubMed] [Google Scholar]
- 3.LinkSchwartz, S. I., Schwartz’s Principles of Surgery. Eleventh edition ed. (2019).
- 4.Karimi, P. et al. Gastric cancer: Descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol. Biomark. Prev.23(5), 700–713 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jun, J. K. et al. Effectiveness of the Korean national cancer screening program in reducing gastric cancer mortality. Gastroenterology152(6), 1319-1328.e7 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Lee, E. K. et al. Improvement of diabetes and hypertension after gastrectomy: A nationwide cohort study. World J. Gastroenterol.21(4), 1173–1181 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phillips, B. T. & Shikora, S. A. The history of metabolic and bariatric surgery: Development of standards for patient safety and efficacy. Metabolism79, 97–107 (2018). [DOI] [PubMed] [Google Scholar]
- 8.Lee, T. H. et al. Long-term follow-up for type 2 diabetes mellitus after gastrectomy in non-morbidly obese patients with gastric cancer: The legitimacy of onco-metabolic surgery. J. Gastric Cancer17(4), 283–294 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim, W. J. et al. Oncometabolic surgery: Emergence and legitimacy for investigation. Chin. J. Cancer Res.32(2), 252–262 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Page, M. J. et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev.10(1), 89 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cumpston, M. et al. Updated guidance for trusted systematic reviews: A new edition of the cochrane handbook for systematic reviews of interventions. Cochrane Database Syst. Rev.10.1002/14651858.ED000142 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Heart, L. and Blood Home, Study Quality Assessment Tools. National Heart, Lung, and Blood Home (2021).
- 13.Chapter 8: Assessing risk of bias in a randomized trial. Cochrane Handbook for Systematic Reviews of Interventions (2022).
- 14.Choi, Y. S. et al. Oncometabolic surgery in gastric cancer patients with type 2 diabetes. Sci. Rep.12(1), 11853 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang, K. C. et al. Outcome after curative surgery for gastric cancer patients with type 2 diabetes. World J. Surg.38(2), 431–438 (2014). [DOI] [PubMed] [Google Scholar]
- 16.Kim, J. H. et al. Multicenter results of long-limb bypass reconstruction after gastrectomy in patients with gastric cancer and type II diabetes. Asian J. Surg.43(1), 297–303 (2020). [DOI] [PubMed] [Google Scholar]
- 17.Kim, J. W. et al. Outcome after gastrectomy in gastric cancer patients with type 2 diabetes. World J. Gastroenterol.18(1), 49–54 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peng, D. et al. Onco-metabolic surgery: A combined approach to gastric cancer and hypertension. Cancer Manag. Res.12, 7867–7873 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiong, S. W. et al. Comparison of different gastric bypass procedures in gastric carcinoma patients with type 2 diabetes mellitus. World J. Gastroenterol.20(48), 18427–18431 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pak, J. et al. Impact of gastrointestinal bypass on nonmorbidly obese type 2 diabetes mellitus patients after gastrectomy. Surg. Obes. Relat. Dis.11(6), 1266–1272 (2015). [DOI] [PubMed] [Google Scholar]
- 21.Ho, T. W. et al. Total gastrectomy improves glucose metabolism on gastric cancer patients: A nationwide population-based study. Surg. Obes. Relat. Dis.12(3), 635–641 (2016). [DOI] [PubMed] [Google Scholar]
- 22.Guner, A. et al. Improved glycemic control with proximal intestinal bypass and weight loss following gastrectomy in non-obese diabetic gastric cancer patients. Oncotarget8(61), 104605–104614 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu, Z. et al. Clinical course of diabetes after gastrectomy according to type of reconstruction in patients with concurrent gastric cancer and type 2 diabetes. Obes. Surg.25(4), 673–679 (2015). [DOI] [PubMed] [Google Scholar]
- 24.Lee, W. et al. Comparative study of diabetes mellitus resolution according to reconstruction type after gastrectomy in gastric cancer patients with diabetes mellitus. Obes. Surg.22(8), 1238–1243 (2012). [DOI] [PubMed] [Google Scholar]
- 25.Kang, K. C. et al. Influence of gastrectomy for stomach cancer on type 2 diabetes mellitus for patients with a body mass index less than 30 kg/m(2). J. Kor. Surg. Soc.82(6), 347–355 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin, X. H. et al. The long term effect of metabolic profile and microbiota status in early gastric cancer patients after subtotal gastrectomy. PLoS One13(11), e0206930 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim, Y. N. et al. Comparison of postoperative nutritional status after distal gastrectomy for gastric cancer using three reconstructive methods: A multicenter study of over 1300 patients. J. Gastrointest. Surg.24(7), 1482–1488 (2020). [DOI] [PubMed] [Google Scholar]
- 28.Kwon, Y. et al. The foregut theory as a possible mechanism of action for the remission of type 2 diabetes in low body mass index patients undergoing subtotal gastrectomy for gastric cancer. Surg. Obes. Relat. Dis.10(2), 235–242 (2014). [DOI] [PubMed] [Google Scholar]
- 29.Wei, Z.-W. et al. Impact of pre-existing type-2 diabetes on patient outcomes after radical resection for gastric cancer: A retrospective cohort study. Dig. Dis. Sci.59(5), 1017–1024 (2014). [DOI] [PubMed] [Google Scholar]
- 30.Tanaka, K. et al. Visceral fat changes after distal gastrectomy according to type of reconstruction procedure for gastric cancer. World J. Surg. Oncol.11(1), 146 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park, Y. S. et al. Nutritional safety of oncometabolic surgery for early gastric cancer patients: A prospective single-arm pilot study using a historical control group for comparison. Surg. Endosc.34(1), 275–283 (2020). [DOI] [PubMed] [Google Scholar]
- 32.Liu, T. et al. Radical gastrectomy combined with modified gastric bypass surgery for gastric cancer patients with type 2 diabetes. Cell Biochem. Biophys.72(3), 839–844 (2015). [DOI] [PubMed] [Google Scholar]
- 33.Park, J. Y. et al. Impact of the different biliopancreatic limb length on diabetes and incretin hormone secretion following distal gastrectomy in gastric cancer patients. Sci. Rep.11(1), 22451 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.An, J. Y. et al. Improvement of type 2 diabetes mellitus after gastric cancer surgery: short-term outcome analysis after gastrectomy. World J. Gastroenterol.19(48), 9410–9417 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hayashi, S. Y. et al. Does Roux-en-Y gastrectomy for gastric cancer influence glucose homeostasis in lean patients?. Surg. Endosc.27(8), 2829–2835 (2013). [DOI] [PubMed] [Google Scholar]
- 36.Kim, J. W. et al. The effect of long Roux-en-Y gastrojejunostomy in gastric cancer patients with type 2 diabetes and body mass index < 35 kg/m(2): preliminary results. Ann. Surg. Treat Res.88(4), 215–221 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang, X. J. et al. Short-term glucose metabolism and gut hormone modulations after Billroth II gastrojejunostomy in nonobese gastric cancer patients with type 2 diabetes mellitus, impaired glucose tolerance and normal glucose tolerance. Arch. Med. Res.44(6), 437–443 (2013). [DOI] [PubMed] [Google Scholar]
- 38.Kim, H. J. et al. Effect of gastrectomy on blood pressure in early gastric cancer survivors with hypertension. Support Care Cancer27(6), 2237–2245 (2019). [DOI] [PubMed] [Google Scholar]
- 39.Lee, Y. K. et al. Metabolic effects of gastrectomy and duodenal bypass in early gastric cancer patients with T2DM: A prospective single-center cohort study. J. Clin. Med.10(17), 4008 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim, W. S. et al. Resolution of type 2 diabetes after gastrectomy for gastric cancer with long limb Roux-en Y reconstruction: A prospective pilot study. J. Kor. Surg. Soc.84(2), 88–93 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park, M. J. et al. Impact of preoperative visceral fat proportion on type 2 diabetes in patients with low body mass index after gastrectomy. Surg. Obes. Relat. Dis.13(8), 1361–1368 (2017). [DOI] [PubMed] [Google Scholar]
- 42.Tanaka, K. et al. Impact of reconstruction method on visceral fat change after distal gastrectomy: results from a randomized controlled trial comparing Billroth I reconstruction and roux-en-Y reconstruction. Surgery155(3), 424–431 (2014). [DOI] [PubMed] [Google Scholar]
- 43.Choi, Y. Y., Noh, S. H. & An, J. Y. A randomized controlled trial of Roux-en-Y gastrojejunostomy versus gastroduodenostomy with respect to the improvement of type 2 diabetes mellitus after distal gastrectomy in gastric cancer patients. PLoS One12(12), e0188904 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Turrentine, F. E. et al. Surgical risk factors, morbidity, and mortality in elderly patients. J. Am. Coll. Surg.203(6), 865–877 (2006). [DOI] [PubMed] [Google Scholar]
- 45.Park, S. H., Kim, J. M. & Park, S. S. Current status and trends of minimally invasive gastrectomy in Korea. Medicina (Kaunas)57(11), 1195 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cummings, D. E. et al. Role of the bypassed proximal intestine in the anti- diabetic effects of bariatric surgery. Surg. Obes. Relat. Dis.3(2), 109–115 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li, J. F. et al. Comparison of laparoscopic Roux-en-Y gastric bypass with laparoscopic sleeve gastrectomy for morbid obesity or type 2 diabetes mellitus: A meta-analysis of randomized controlled trials. Can. J. Surg.56(6), E158–E164 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Batterham, R. L. & Cummings, D. E. Mechanisms of diabetes improvement following bariatric/metabolic surgery. Diab. Care39(6), 893–901 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kwon, O. et al. The recovery of beta-cell function is critical for antidiabetic outcomes of gastric bypass in asian subjects with type 2 diabetes and a body mass index below 30. Obes. Surg.27(2), 541–544 (2017). [DOI] [PubMed] [Google Scholar]
- 50.Lee, W. J. et al. Predicting success of metabolic surgery: Age, body mass index, C-peptide, and duration score. Surg. Obes. Relat. Dis.9(3), 379–384 (2013). [DOI] [PubMed] [Google Scholar]
- 51.Kwon, Y. et al. Predictors of remission and relapse of diabetes after conventional gastrectomy for gastric cancer: Nationwide population-based cohort study. J. Am. Coll. Surg.232(6), 973-981.e2 (2021). [DOI] [PubMed] [Google Scholar]
- 52.Kunisaki, C. et al. Significance of long-term follow-up of early gastric cancer. Ann. Surg. Oncol.13(3), 363–369 (2006). [DOI] [PubMed] [Google Scholar]
- 53.Lee, Y. H. et al. Gastrectomy for early gastric cancer is associated with decreased cardiovascular mortality in association with postsurgical metabolic changes. Ann. Surg. Oncol.20(4), 1250–1257 (2013). [DOI] [PubMed] [Google Scholar]
- 54.Fujioka, S. et al. Contribution of intra-abdominal fat accumulation to the impairment of glucose and lipid metabolism in human obesity. Metabolism36(1), 54–59 (1987). [DOI] [PubMed] [Google Scholar]
- 55.Lordick, F. et al. Gastric cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann. Oncol.33(10), 1005–1020 (2022). [DOI] [PubMed] [Google Scholar]
- 56.Sekiguchi, M. et al. Epidemiological trends and future perspectives of gastric cancer in Eastern Asia. Digestion103(1), 22–28 (2022). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article and its supplementary information files.