Abstract
OBJECTIVE
To investigate the role of satellite glial cells in irritable bowel syndrome (IBS) and the effect of electroacupuncture (EA) at the Tianshu (ST25) and Shangjuxu (ST37) combination.
METHODS
A model for visceral hypersensitivity in IBS was induced through colorectal distension (CRD) stimulation. Clean-grade male Sprague-Dawley (SD) rats were randomly divided into four groups: a normal group (NG), a model group (MG), an electroacupuncture group (EA), and a glial cell inhibitor group (FCA). Bilateral EA (2/100 Hz, 1 mA, 30 min) was administered at the Tianshu (ST25) and Shangjuxu (ST37) in week 6. Abdominal withdrawal reflex (AWR) scores were used to assess the behavioral response associated with visceral hyperalgesia, while hematoxylin-eosin staining was employed to evaluate pathological changes in the colon. The protein and mRNA levels of glial fibrillary acidic protein (GFAP) in the colon and colon-related dorsal root ganglion (DRG) were analyzed using immun-ofluorescence, immun-ohistochemistry, Western blotting, real-time polymerase chain reaction. The impact of EA on electrophysiological properties of colon-related DRG neurons was observed through whole-cell patch clamp analysis.
RESULTS
EA significantly reduced the visceral pain behavior scores in rats with IBS in response to graded (20, 40, 60, 80 mm Hg) CRD stimulation. Additionally, EA downregulated the protein and mRNA expression levels of GFAP in the colon and colon-related DRG of rats with IBS. EA also regulated the resting membrane potential, rheobase and action potential of colon-related DRG neurons in rats with IBS.
CONCLUSIONS
EA can regulate the excitatory properties of colon-related DRG neurons by downregulating the protein and mRNA expression of GFAP in the colon and colon-related DRG, indicating a potential neurobiological mechanism by which EA relieves visceral hypersensitivity in rats with IBS.
Keywords: electroacupuncture, satellite glial cell, irritable bowel syndrome, visceral hypersensitivity, glial fibrillary acidic proteinSupporting information
1. INTRODUCTION
Irritable bowel syndrome (IBS) is a common, chronic, recurring, and remitting functional disorder of the gastrointestinal (GI) tract. It is characterized by abdominal pain or discomfort, changes in bowel habits, and a persistent or intermittent onset without a known structural or anatomical explanation.1,2 These clinical characteristics lead to substantial disruptions in the life and work of patients. However, the etiology and pathogenesis of IBS remain unclear. Presently, IBS is considered to be mainly related to visceral hypersensitivity, GI motility disorders, intestinal infection-related factors, psycho-psychological and genetic factors, and brain-intestinal axis disorders.3,4 Recent studies have considered visceral hypersensitivity as an important pathophysiological characteristic of IBS.
Visceral hypersensitivity is defined as the presence of low thresholds for stimuli perception in the gut, leading to increased responsiveness to various mechanical and chemical injurious stimuli. This phenomenon is characterized by two components: hyperalgesia, an enhanced response to a painful stimulus, and allodynia, a painful response to an innocuous stimulus.5,6 Both clinical and animal studies have confirmed that visceral hypersensitivity plays a crucial role in visceral pain associated with IBS.7 The pathogenesis of visceral hypersensitivity is complex, involving all levels of the brain-gut axis, both the peripheral and central nervous systems.
Growing evidence implicates glial cells in the development and maintenance of chronic pain in addition to nutritional support for neurons.8 A study on the pathological mechanism of visceral pain revealed that glial cells promoted the occurrence of IBS-related abdominal pain.9 Glial cells, mainly astrocytes and microglia, are involved in the transmission and transduction of nociceptive signals through various pathways.10 Enteric glial cells (EGCs) are satellite cells of the GI tract sensory nerves and sympathetic ganglia in the enteric nervous system (ENS). These cells play a crucial role in nutrition and the regulation of intestinal nerves, the coordination of intestinal movement, and the maintenance of intestinal homeostasis.11 Additionally, EGCs participate in the regulation of intestinal immunity and barrier defense function. Notably, EGCs are similar to astrocytes of the central nervous system (CNS), and glial fibrillary acidic protein (GFAP) is a marker for their expression and activation. Purinergic 2 X and Purinergic 2 Y receptors existing on the surface of astrocytes, which can facilitate the entry of Ca2+ into the cytoplasm through ion channels on the membrane and release Ca2+ from the intracellular calcium stores into the cytoplasm.12,13 The increase in Ca2+ concentration is one of the important mechanisms for the proliferation, migration and release of neurotransmitters in astrocytes.14 DL-Fluorocitric acid barium salt (FCA) can selectively inhibit the tricarboxylic acid cycle of glucose metabolism in glial cells, thus inhibiting their activity.15 The function of glial cells in mediating pain has garnered increasing attention through intensive research on their involvement in the occurrence and maintenance of pain sensation at all levels of the nervous system.
Acupuncture and moxibustion, integral therapies in Traditional Chinese Medicine, regulate Zang and Fu, dredge meridians and collaterals, strengthen healthy Qi, eliminate pathogenic factors, and harmonize Qi and blood. Guided by the holistic principles of Traditional Chinese Medicine, along with syndrome differentiation and treatment, these therapies incorporate the four diagnostic methods and the combination of disease and syndrome. Acupuncture and moxibustion have demonstrated significant efficacy in the treatment of IBS. Our previous clinical and basic studies have confirmed the effectiveness of acupuncture and moxibustion in the treatment of IBS.16,17 We found that EA has an excellent analgesic effect on IBS-related visceral pain. This analgesic effect involves peripheral, central and other levels.18,19 This present study was performed to investigate the peripheral effect of EA on visceral hypersensitivity in rats with IBS. Astrocytes were found to be involved in the analgesic activity of EA. From this perspective, experimental evidence of acupuncture regulating the mechanism of peripheral sensitization was obtained, followed by an in-depth analysis of astrocytes in the colon and colon-related DRG (T13-L2 and L6-S2 segments) during the treatment of IBS-related visceral hypersensitivity with EA.
2. MATERIALS AND METHODS
2.1. Animals
Male Sprague-Dawley (SD) rats (at four days of age) of Clean grade were supplied by the Animal Experimental Center of Shanghai University of Traditional Chinese Medicine [Animal license number: SCXK (Shanghai) 2013-0016]. Before the experiments, the rats were fed for 3 d to enable them to adapt to the environment. Each litter had 6 to 7 neonatal rats. All litters of rat pups were kept with a lactating female rat, and the lactating rats were given food and water ad libitum. The rat housing environment was as follows: 12∶12 dark-light cycle, room temperature of (20 ± 2) ℃, and indoor humidity of 50%-70%. The whole experimental protocol was approved by the Animal Ethics Committee of Shanghai University of Traditional Chinese Medicine (permit number: PZSHUTCM200417003) and performed according to the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines.20
2.2. Induction of visceral hypersensitivity
Eight-day-old neonatal rats were randomly divided into a normal group (NG) and a model group (MG). The neonatal rats in NG did not receive any treatment, but those in MG were subjected to colorectal distention (CRD) stimulation in an awake state. The surgical procedure to establish the chronic visceral hypersensitivity rat model was according to the process described by Al-Chaer et al.21 During the procedure, the surface of a balloon was first lubricated with an appropriate amount of liquid paraffin and then slowly inserted through the anus along the physiological rectal curvature of the neonatal rats to a depth of approximately 2 cm, until the balloon reached the descending colon. The balloon was inflated to approximately 0.2 mL using a syringe for 1 min, followed by deflation, and the same stimulation was repeated once after 1 h. After the end of stimulation, the balloon was removed. This procedure was performed once a day for 14 d. Two weeks after the end of the CRD stimulation, the abdominal withdrawal reflex (AWR) was assessed.
2.3. Visceromotor responses (VMRs) to CRD
AWR was assessed within 2 h after the end of the intervention on the 36th and 43rd days, following the experimental procedure described by Al-Chaer et al.20 To reduce fecal formation before AWR assessment, the rats were fasted (8-12 h) but still had access to water. The CRD stimulation method was as follows: a homemade balloon stimulator, a medical sphygmomanometer and a syringe were connected via a 3-way valve; the balloon was inserted into the descending colon from the anus along the physiological curvature of the rectum in awake rats; and colonic stimulation then conducted via constant pressure dilatation at 20, 40, 60, 80 mm Hg. AWR scores were measured 3 times for each rat under each pressure. Each stimulation lasted for approximately 20 s, with 1-min interval between repetitions at the same pressure and a 4-min interval between different pressures. The mean value was taken as the final score. The standard for AWR score was based on the behavioral scale of AWR described by Al-Chaer et al.20
2.4. EA and drug administration
Based on a confirmed successful model, in the 6th week after the procedure, rats in the model group were randomly divided into three groups: a model group (MG), an electroacupuncture group (EA), and a FCA group. The rats in the NG and MG received the same fixation as those in the EA group. For the EA group, rats were secured in a rat fixation frame, and a black cover was placed over their heads to maintain tranquility. Subsequently, the rats underwent EA stimulation, involving the insertion of acupuncture needles (0.18 mm × 13 mm; Huatuo, Suzhou Medical Co., Ltd., Suzhou, China) at bilateral Tianshu (ST25, 0.2 cm lateral to the intersection of upper 8/13 and lower 5/13 from xiphoid to symphysis) and bilateral Shangjuxu (ST37, intersection of upper 6/16 and lower 10/16 of lateral condyle of tibia and lateral malleolus, 0.1 cm lateral to crista anterior tibiae)22 to a depth of approximately 5 mm. After insertion, the needle handles were connected to Han’s acupoint nerve stimulator (HANS-100A, Nanjing Jisheng Medical Technology Co., Ltd., Nanjing, China) and stimulated for 30 min at an alternating frequency of 2/100 Hz and an intensity of 1 mA. The EA stimulation was conducted once a day for 7 d. The rats in the FCA group received an intrathecal injection of FCA (10 μL, 1 nmol/μL, F9634, Sigma, St. Louis, MO, USA)9 every 3 d (days 1, 3, 7).
2.5. Hematoxylin & eosin (HE) staining
Sections of the colon tissue (4 µm thick) were deparaffinized by immersion in xylene (15 min × 2) and rehydrated using a graded ethanol series (100% for 5 min; 90% for 5 min; 80% for 5 min; and 70% for 5 min). Next, the sections were stained with hematoxylin solution for 1 min, followed by 2 immersions in 1% acid ethanol before rinsing under running water for 10 min. The sections were then stained with eosin solution for 5 min, dehydrated in graded alcohol (70% for 1 min; 80% for 1 min; 90% for 2 min; and 100% for 2 min), and cleared in xylene (15 min × 2). The mounted sections were then examined and photographed using an Olympus BX53 microscope (Tokyo, Japan).
2.6. Immunofluorescence of the intestinal plexus
The colon tissues, fixed with 4% paraformaldehyde were dehydrated overnight with a 30% sucrose solution. Subsequently, the intestinal plexuses were obtained after removing the mucosal layer, submucosal, circular muscle layer, serosal layer, and longitudinal muscle layers under a microscope. The intestinal plexuses were then spread onto slides. After rehydration, endogenous peroxidases were inhibited by incubation in 0.3% H2O2 in methanol for 20 min, followed by washing (5 min × 2) with distilled water. The slides were blocked with goat serum for 20 min at room temperature, followed by overnight incubation at 4 ℃ with an anti-GFAP antibody [1∶1000 in Tris-buffered saline (TBS); ab7260, Abcam, Cambridge, UK]. The next day, the slides were rinsed with TBS (5 min × 3) and incubated with goat anti-rabbit immunoglobulin G (IgG) (H+L) secondary antibody (fluoresceine isothiocyanate conjugate; 1∶32 in TBS; BA1105, Boster, Wuhan, China) for 30 min at room temperature without light. Following this, the slides were washed in TBS (5 min × 3). The slides were then mounted, examined, and photographed using an Olympus BX53 fluorescence microscope (Tokyo, Japan).
2.7. Immunohistochemistry
After deparaffinization and rehydration, endogenous peroxidases were inhibited by incubation in 0.3% H2O2 in methanol for 20 min, followed by washing (5 min × 2) with distilled water. The samples were then spread onto slides, which were blocked with goat serum for 20 min at room temperature, followed by overnight incubation at 4 ℃ with an anti-GFAP antibody [1∶1000 in phosphate-buffered saline (PBS); ab7260, Abcam, Cambridge, UK]. The next day, the slides were rinsed with PBS (5 min × 3) and incubated with goat anti-rabbit IgG (H + L) secondary antibody (biotin conjugate; 1∶100 in PBS; BA1003, Boster, Wuhan, China) for 30 min at room temperature. Following this, the slides were rinsed with PBS (5 min × 3) and subsequently stained with diaminobenzidine (DAB) and hematoxylin. The slides were then examined and photographed using an Olympus BX53 microscope (Tokyo, Japan). The DAB staining intensity was analyzed using the Image-Pro Plus 6.0 software (Media Cybernetics, Rockville, MD, USA) and expressed as integrated optical density values.
2.8. Western blotting
Total protein was extracted from colon and colon-related DRG tissues using radioimmunoprecipitation assay (RIPA) lysis buffer (P0013B, Beyotime Biotechnology, Shanghai, China) and phenylmethanesulfonyl fluoride (ST506, Beyotime Biotechnology, Shanghai, China). Protein concentration was determined using the bicinchoninic acid (BCA) protein assay kit (P0012, Beyotime Biotechnology, Shanghai, China). The samples were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto a nitrocellulose (NC) membrane (wet transfer). Then, the NC membrane was blocked at room temperature with 5% bovine serum albumin (BSA) on a shaker. Next, the membrane was incubated with an anti-GFAP antibody (1∶1000 in 5% BSA; ab7260, Abcam, Cambridge, UK) and rabbit anti-β-actin (13E5) mAb (1∶1000 in 5% BSA; 4970S, CST, Boston, BSN, USA), followed by incubation with a horseradish peroxidase-labeled goat anti-rabbit IgG secondary antibody (1∶1000 in 5% PBS; A0208, Beyotime Biotechnology, Shanghai, China). Finally, the target protein bands were visualized using the BeyoECL Plus kit (P0018, Beyotime Biotechnology, Shanghai, China).
2.9. Real-time polymerase chain reaction
Total RNA was extracted from colon and colon-related DRG tissue homogenates using TRIzol (15596018, Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol and quantified by spectrophotometry. Sample concentrations, purities and integrities were also determined. A total RNA equivalent was used to reverse transcribe cDNA via real-time PCR using the SYBR Green PCR kit (208052, Qiagen, Dusseldorf, Germany) and cDNA synthesis kit (K1662, Invitrogen, Carlsbad, CA, USA). The ABI Prism 7500 SDS software (ABI, Carlsbad, CA, USA) was used to analyze the mRNA expression levels of target genes. The sequence-specific primers used were as follows: GFAP (Fw) 5’-TCAATGCCGGCTTCAAAGAG-3’ and (Rev) 5’-TTCCAGGAAGCGGACCTTCT-3’; and glyceral-dehyde-3-phosphate Dehydrogenase (GAPDH) (Fw) 5’-GGCAAGTTCAACGGCACAGT-3’ and (Rev) 5’-ATGACATACTCAGCACCGGC-3’. The relative mRNA expression was normalized to GAPDH and analyzed using the 2−ΔΔCT method. All experiments were repeated at least 3 times.
2.10. Dissociation of DRG neurons
The previously extracted DRGs (L6 segment) were placed in an oxygen-permeable extracellular fluid at 4 ℃. The outer membrane covering the surface was peeled off with the aid of a dissection microscope. The sample was then digested in 1 mL of digestion solution containing 1 mg/mL trypsin (25200-056, Gibco, Grand Island, NY, USA), 1.5 mg/mL type Ⅱ collagenase (LS004176, Worthington, Lakewood, NJ, USA) and 6 mg/mL BSA (ST023, Beyotime Biotechnology, Shanghai, China) for 45 min at 37 ℃. After digestion, the mixture was gently pipetted to generate a single-cell suspension and centrifuged (300 g, 2 min, 4 ℃). The supernatant was discarded, and the pellets were resuspended in an appropriate amount of oxygen-saturated extracellular fluid and finally placed onto slides. The cells were allowed to adhere to the slides, and after 1 h, the slides were used for patch clamp recording.
2.11. Patch clamp recording
The prepared slides with cells were placed in an oxygen-saturated extracellular fluid [150 mM NaCl, 5 mM KCl, 2.5 mM CaCl2, 1 mM MgCl2·6H2O; 10 mM glucose; 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), pH adjusted to 7.4 with NaOH] and transferred onto a patch clamp table for recording. Smooth and plump cells were selected as experimental samples. The pipette used was equipped with a P-1000 pipette-pulling device, and its tip had a diameter ranging from 1 to 2 μm in diameter and filled with the pipette solution (140 mM KCl, 1 mM MgCl2·6H2O, 5 mM ethylene glycol tetraacetic acid, 3 mM Na2ATP, 0.2 mM Na3GTP, 10 mM HEPES, pH adjusted to 7.2 with KOH), and the impedance was 3 to 5 MΩ. The recording frequency was 1 kHz, the acquisition frequency was 10 kHz, and the clamping voltage was -70 mV. In the current clamp mode, a current stimulus with an intensity of -200 to 200 pA was applied. The minimum stimulation current that caused the cell to generate an action potential (AP) was used as the rheobase, and the resting membrane potential (RMP) value, the rheobase value, and the AP frequency were recorded.
2.12. Statistical analysis
Statistical analyses of all experimental data were performed using the SPSS 24.0 statistical software (IBM Corp., Armonk, NY, USA). If the quantitative data were subjected to the normality test and the homogeneity of variance test, the differences between groups were compared using one-way analysis of variance (ANOVA). The least significant difference method was employed for comparisons between groups in the case of homogeneity of variance, while Dunnett’s T3 test was used for comparisons between groups in the case of heterogeneity of variance. If the data were not normally distributed, the Kruskal-Wallis H test was performed after rank transformation. P<0.05 was considered statistically significant.
3. RESULTS
3.1. EA relieves visceral hypersensitivity
AWR scores were obtained before the intervention on rats on the 36th day. The AWR scores of the MG were significantly higher than those of the NG with CRD stimulation of 20, 40 and 60 mm Hg (all P<0.01, Table 1). The AWR score of the MG was also higher than that of the NG with CRD stimulation of 80 mm Hg (Table 1), although this difference was not statistically significant. Thus, the visceral hypersensitivity model of IBS was successfully established.
Table 1.
AWR scores before EA and drug intervention [M (Q25, Q75)]
| Group | n | 20 mm Hg | 40 mm Hg | 60 mm Hg | 80 mm Hg |
|---|---|---|---|---|---|
| NG | 8 | 0.33 (0, 0.33) | 1.33 (1.33, 1.33) | 2.67 (2.67, 2.67) | 3.84 (3.33, 4) |
| MG | 24 | 0.67 (0.33, 1.00)a | 2.33 (2, 2.67)a | 3.33 (3, 3.33)a | 4 (3.67, 4) |
Notes: NG: normal group, the rats in NG did not receive any treatment; MG: model group, the rats in MG were subjected to colorectal distention stimulation for 2 weeks in an awake state. Kruskal-Wallis H test was used to analysis the AWR scores before EA and drug intervention. AWR: abdominal withdrawal reflex; EA: electroacupuncture. Compared with the normal group, aP<0.01.
AWR scores were also obtained collected after the intervention on rats on the 43rd day. Notably, the AWR scores of the MG were significantly higher than those of the NG with graded CRD (all P<0.05, Table 2). The AWR scores of the EA and FCA groups were significantly lower than those of the MG with graded CRD (all P<0.05, Table 2). However, there was no statistically significant difference in AWR score between the EA and FCA groups (Table 2). Therefore, it can be concluded that EA has the potential to reduce the visceral hypersensitivity of rats with IBS, and FCA reduces visceral hypersensitivity by inhibiting the expression of glial cells. Both EA at the Shangjuxu (ST37) and Tianshu (ST25) acupoints and intrathecal FCA injection could thus reduce the chronic visceral hypersensitivity of rats with IBS by increasing the pain threshold.
Table 2.
AWR scores after EA and drug intervention [M (Q25, Q75)]
| Group | n | 20 mm Hg | 40 mm Hg | 60 mm Hg | 80 mm Hg |
|---|---|---|---|---|---|
| NG | 8 | 0.33 (0, 0.33) | 1.5 (1.33, 1.67) | 2.33 (2.08, 2.59) | 3.33 (3.08, 3.67) |
| MG | 8 | 0.67 (0.67, 0.92)a | 2 (2, 2.33)a | 3 (2.75, 3.25)a | 4 (3.67, 4)a |
| EA | 8 | 0.33 (0.08, 0.67)b | 2 (1.67, 2)b | 2.67 (2.67, 2.92)b | 3.5 (3.08, 3.92)b |
| FCA | 8 | 0.17 (0, 0.33)c | 2 (1.67, 2)b | 2.67 (2.42, 2.92)b | 3.33 (3.33, 3.59)b |
Notes: NG: normal group, the same fixation as the EA group; MG: model group, the same fixation as the EA group; EA: electroacupuncture group, EA stimulation at bilateral Tianshu (ST25) and Shangjuxu (ST37) (2/100 Hz, 1 mA, 30 min, 7 d); FCA: DL-fluorocitric acid barium salt group, intrathecal injection of FCA (10 μL, 1 nmol/μL) every three days. Kruskal-Wallis H test was used to analysis the AWR scores after EA and drug intervention. AWR: abdominal withdrawal reflex; EA: electroacupuncture. Compared with the normal group, aP<0.01; compared with the model group, bP<0.05, cP<0.01.
3.2. Pathology of colon tissues (HE staining)
In the NG, the mucosal epithelium was intact, and the glands in the lamina propria were arranged in an ordered fashion. There was no significant inflammatory cell infiltration and no noticeable interstitial edema. Similarly, in the MG, the mucosal epithelium was intact, and the glands in the lamina propria were arranged in an ordered fashion. There was a small degree of inflammatory cell infiltration and interstitial edema. In the EA group, the mucosal epithelium was intact, and the glands in the lamina propria were arranged in an ordered fashion. There was no significant inflammatory cell infiltration, but there was mild interstitial edema. In the FCA group, the mucosal epithelium was intact, and the glands in the lamina propria were arranged in an ordered fashion. There was no significant inflammatory cell infiltration, but there was mild interstitial edema (Figures 1A-1D). These findings are consistent with the clinical characteristics of IBS, although there were no noticeable histopathological changes.
Figure 1. HE staining of colon tissues.
A-D: HE staining of colon tissues, scale bar: 100 μm. A: NG; B: MG; C: EA; D: FCA. NG: normal group, the same fixation as the EA group; MG: model group, the same fixation as the EA group; EA: electroacupuncture group, EA at bilateral Tianshu (ST25) and Shangjuxu (ST37) (2/100 Hz, 1 mA, 30 min, 7 d); FCA: DL-fluorocitric acid barium salt group, intrathecal injection of FCA (10 μL, 1 nmol/μL) every three days. HE: hematoxylin and eosin.
3.3. Protein and mRNA expression of GFAP in the colon
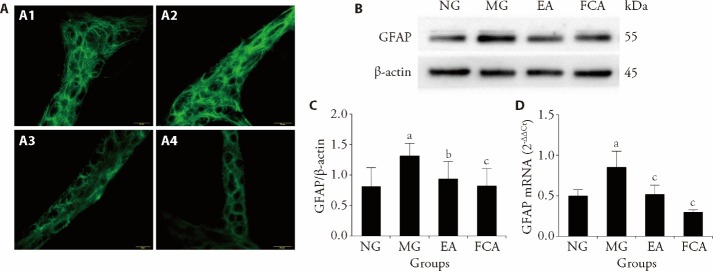
The expression of GFAP was detected in the colonic myenteric plexus of rats in each group, primarily in the glial cells surrounding the intermuscular neurons of the colon. CRD stimulation in neonatal rats upregulated the expression of GFAP in the colonic myenteric plexus (Figure 2A). Moreover, both EA and FCA interventions downregulated the abnormal expression of GFAP in the colonic myenteric plexus of these rats (Figure 2A).
Figure 2. GFAP expression in the colonic myenteric plexus and colon.
A: immunofluorescence was used to detect the distribution of GFAP in the colonic myenteric plexus, scale bar: 20 μm; A1: NG; A2: MG; A3: EA; A4: FCA. B: representative gel images show the protein level of GFAP in the colon, β-Actin was used as a loading control; Western blotting was used to detect the expression of GFAP protein in the colon. C: quantitative analysis of GFAP protein expression in the colon. D: relative expression of GFAP mRNA in the colon, real-time polymerase chain reaction was used to detect the expression of GFAP mRNA in the colon. NG (n = 8): normal group, the same fixation as the EA group; MG (n = 8): model group, the same fixation as the EA group; EA (n = 8): electroacupuncture group, EA stimulation at bilateral Tianshu (ST25) and Shangjuxu (ST37) (2/100 Hz, 1 mA, 30 min, 7 d); FCA (n = 8): DL-fluorocitric acid barium salt group, intrathecal injection of FCA (10 μL, 1 nmol/μL) every three days. GFAP: glial fibrillary acidic protein; mRNA: messenger ribonucleic acid. All data was easured by one-way analysis, and least significance difference test was performed for inter-group comparisons. All data was presented as mean ± standard deviation. Compared with the normal group, aP<0.01; compared with the model group, bP<0.05, cP<0.01.
Further examination of the protein and mRNA expression of GFAP in the colon revealed that CRD stimulation in neonatal rats induced an increase in the protein and mRNA expression of GFAP in the colon of rats with visceral hypersensitivity (P<0.01, Figures 2B-2D). Additionally, both EA and FCA intervention downregulated the protein and mRNA expression of GFAP in the colon of rats with visceral hypersensitivity (P<0.01, Figures 2B-2D). These findings are consistent with the previously observed expression of GFAP in the colonic myenteric plexus, suggesting that EA relieves visceral pain by regulating the protein and mRNA expression of GFAP in the colon.
3.4. Protein and mRNA expression of GFAP in the colon-related DRGs
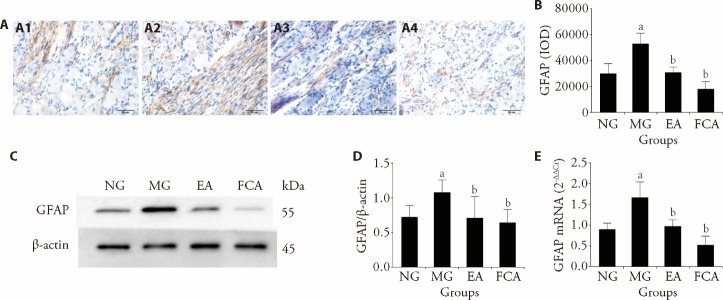
Astrocytes are mainly present around neurons of the DRG. The distribution of GFAP was further assessed by immunohistochemistry. The positive expression of GFAP on astrocytes was indicated by a yellowish-brown stain. In the NG, the positively stained astrocytes were uniformly distributed and the staining intensity was faint, indicating a negative or weakly positive signal. However, some axons were stained more deeply than others. In the MG, the positively stained astrocytes were distributed more densely and the staining intensity was deep, indicating a positive or strongly positive signal. Additionally, the axons were stained more deeply. In the EA and FCA groups: The positively stained astrocytes were distributed more sparsely and the staining intensity was faint, indicating a negative or weakly positive signal. However, some axons were stained more deeply than others (Figure 3A). Semiquantitative analysis revealed that CRD stimulation in neonatal rats induced an increase in the positive expression of GFAP in the colon-related DRG of rats with visceral hypersensitivity (P<0.01, Figure 3B). Additionally, both EA and FCA interventions downregulated the positive expression of GFAP in the colon-related DRG of rats with visceral hypersensitivity (P<0.01, Figure 3B).
Figure 3. GFAP protein and mRNA expression in the colon-related DRG.
A: immunohistochemical assay was used to detect the expression of GFAP protein in the colon-related DRG, scale bar: 50 μm; A1: NG; A2: MG; A3: EA; A4: FCA; dyeing method of all pictures are the immunohistochemical DAB method. B: semiquantitative analysis of GFAP protein expression in the colon-related DRG. C: representative gel images show the protein level of GFAP in the colon-related DRG, β-Actin was used as a loading control; Western blotting was used to detect the expression of GFAP protein in the colon-related DRG. D: quantitative analysis of GFAP protein expression in the colon-related DRG. E: relative expression of GFAP mRNA in the colon-related DRG, Real-time Polymerase Chain Reaction was used to detect the expression of GFAP mRNA in the colon. NG (n = 8): normal group, the same fixation as the EA group; MG (n = 8): model group, the same fixation as the EA group; EA (n = 8): electroacupuncture group, EA stimulation at bilateral Tianshu (ST25) and Shangjuxu (ST37) (2/100 Hz, 1 mA, 30 min, 7 d); FCA (n = 8): DL-fluorocitric acid barium salt group, intrathecal injection of FCA (10 μL, 1 nmol/μL) every three days. GFAP: glial fibrillary acidic protein; DAB: diaminobenzidine; IOD: immunohistochemical optical density. mRNA: messenger ribonucleic acid; DRG: dorsal root ganglion. All data was measured by one-way analysis, and least significance difference test was performed for inter-group comparisons. All data was presented as mean ± standard deviation. Compared with the normal group, aP<0.01; compared with the model group, bP<0.01.
Subsequently, we examined this regulatory effect based on the protein and mRNA levels of GFAP using Western blotting and real-time PCR, and the results were consistent with our previous findings. CRD stimulation in neonatal rats induced an increase in the protein and mRNA expression of GFAP in the colon-related DRG of rats with visceral hypersensitivity (P<0.01, Figures 3C-3E). Both EA and FCA interventions downregulated the protein and mRNA expression of GFAP in the colon-related DRG of rats with visceral hypersensitivity (all P <0.05, Figures 3C-3E), suggesting that both EA and FCA relieve visceral pain by regulating the protein and mRNA expression of GFAP in colon-related DRGs.
3.5. EA regulates the excitability of colon-related DRG neurons
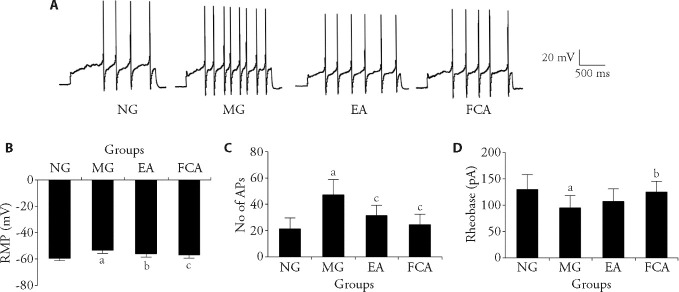
In our previous studies, we found that EA can regulate the excitability of primary sensory neurons in rats with CRD stimulation-induced visceral pain induced, and there is an interaction between neurons and glial cells. This present study aimed to use electrophysiological methods to further explore whether glial cells are involved in the EA-induced inhibition of the peripheral sensitization to visceral pain by EA. We used a whole-cell recording method to monitor changes in the excitability of colon-related DRG neurons. Notably, CRD stimulation significantly upregulated the RMP of colon-related DRG neurons (P<0.01, Figure 4B), increased the number of APs propagated by colon-related DRG neurons (P<0.01, Figure 4C), and downregulated the rheobase of colon-related DRG neurons (P<0.01, Figure 4D). EA inhibited the excitability of colon-related DRG neurons in rats with IBS. This inhibition included lowering the RMP (P<0.05, Figure 4B), inhibiting the propagation of APs (P<0.01, Figure 4C), and upregulating the rheobase of colonic neurons (Figure 4D). Intrathecal FCA injection to inhibit the activity of glial cells resulted in the inhibition of excitability of colon-associated DRG neurons inrats with IBS (P<0.05, Figures 4B-4D). Considering the previous behavioral and molecular biological results, we speculate that glial cells play a crucial role in the peripheral neurobiological mechanism through which EA relieves visceral pain.
Figure 4. Changes in the electrical properties of membrane of the colon-related DRG neurons.
A: representative traces of APs after a 200 pA depolarization current injection into colon-related DRG neurons of the NG, MG, and the EA, and FCA groups; single cell patch clamp recording was used to detect the RMP, rheobase and AP frequency of the colon-related DRG neurons. B: changes in the cell RMP in colon-related DRG neurons; C: number of APs of neuronal cells in colon-related DRG; D: changes in the rheobase in colon-related DRG neurons. NG (n = 8): normal group, the same fixation as the EA group; MG (n = 8): model group, the same fixation as the EA group; EA (n = 8): electroacupuncture group, EA stimulation at bilateral Tianshu (ST25) and Shangjuxu (ST37) (2/100 Hz, 1 mA, 30 min, 7 d); FCA (n = 8): DL-fluorocitric acid barium salt group, intrathecal injection of FCA (10 μL, 1 nmol/μL) every three days. RMP: resting membrane potential; No: number; AP: action potential; DRG: dorsal root ganglion. All data was measured by one-way analysis, and least significance difference test was performed for inter-group comparisons. All data was presented as mean ± standard deviation. Compared with the normal group, aP<0.01; Compared with the model group, bP<0.05, cP<0.01.
4. DISCUSSION
Visceral hypersensitivity is considered one of the main physiological and pathological mechanisms,23 and has been proven to play a key role in IBS-related abdominal pain. In this study, a CRD stimulation-induced visceral hypersensitivity model of IBS was established. This model mainly simulates the visceral hypersensitivity response in IBS caused by mechanical dilation-mediated injury. CRD stimulation was performed on the neonatal rats (8th day of birth), and the measure of AWR score was assessed on the 36th day. Notably, the AWR scores of rats in the MG were higher than those those of rats in the NG with graded CRD stimulation, confirming the induction of visceral hypersensitivity. Subsequent AWR assessments on the 43rd day revealed that AWR scores of rats in the MG were still higher than those of rats in the NG with graded CRD stimulation, confirming the persistence of visceral hypersensitivity in adult rats (Figures 1A, 1B). Pathological examination of colon tissues revealed no obvious changes in specimens from any of the groups, and the mucosal epithelial cells were intact (Figure 1C). These findings are consistent with the pathological manifestations of clinical IBS. Thus, the visceral hypersensitivity model of IBS was successfully established.
At present, there is no specific Western Medicine for treating IBS. However, both clinical and basic studies have confirmed that acupuncture is effective in the treatment of IBS-related abdominal pain.17,19 Acupuncture can relieve pain mainly by stimulating the endogenous analgesia system of the body, integrating the peripheral and central nervous systems to resist the nociceptive stimulus.24 Acupuncture has a significant analgesic effect on all kinds of pain, including IBS-related visceral pain. EA, in particular, has been internationally recognized for its analgesic properties.25 The acupoints Tianshu (ST25) and Shangjuxu (ST36) selected in this study belong to the Stomach Meridian of Foot Yangming. The combination of Tianshu (ST25) and Shangjuxu (ST36) forms the He-Mu point combination, which is a classic and effective point combination for treating abdominal pain, diarrhea, or constipation. When combined, one points can regulate the upward movement of Qi and the other can regulate the downward movement of Qi, synergistically achieving the effects of soothing the Qi of intestine and eliminating the pathogenic factors.26,27 In this study, the AWR scores of rats with IBS-related visceral hypersensitivity induced by CRD stimulation were effectively reduced by EA (Figure 1B), indicating that EA has an analgesic effect on rats with IBS-related visceral hypersensitivity by behavioristics, as demonstrated through behavioral assessments.
The sensory ganglion is the first station of the pain sensory pathway, and DRG is the main type of sensory ganglion that regulates sensory perception in most parts of the body, including the internal organs. The nociceptive information of the colon and rectum is transmitted to the CNS through DRG at the T13-L2 and L6-S2 segments.28 Neuronal sensitization at the sensory ganglion contributes to peripheral sensitization to chronic pain.29,30 When neurons are activated, astrocytes will become activated because of their intimate contact with the neurons. Activated glial cells are characterized by decreased ramification, hypertrophy, and proliferation. The expression of GFAP, a marker of astrocytes activation, may increase with astrocytic activation.31 This study investigated the mechanism underpinning the analgesic effect of acupuncture from the perspective of glial cell activation in the colon and colon-related DRG. We first detected the expression of GFAP in the colonic myenteric plexus of rats with IBS-related chronic visceral pain induced by CRD stimulation using immunofluorescence. We found that the expression of GFAP in the MG was higher, while it was lower in the EA and FCA groups. This suggests that glial cells in the colonic myenteric plexus of rats with IBS-related chronic visceral pain induced by CRD stimulation were activated, and both EA and FCA interventions could inhibit the activity of glial cells activity (Figure 2A). Subsequently, the protein and mRNA expression of GFAP in the colon were analyzed. The findings confirmed that the protein and mRNA expression of GFAP were increased in the colon of rats with IBS-related chronic visceral pain induced by CRD stimulation, and both EA and FCA interventions could decrease the protein and mRNA expression of GFAP in the colon (Figures 2B-2D). A subsequent analysis of the protein and mRNA expression of GFAP in colon-related DRG, revealed that the protein and mRNA expression of GFAP increased in the colon-related DRG of the rats, and both EA and FCA interventions could decrease the protein and mRNA expression of GFAP in the colon-related DRG (Figure 3). To further verify the influence of glial cells in the DRG on the sensitization of neurons, we observed the changes in the electrical properties of the membranes (the resting membrane potential, rheobase and action potential) of DRG neurons in the L6 segment through whole-cell recording. There was an increase in RMP and the number of APs, and a decrease in rheobase of DRG neurons in the L6 segment of rats with IBS-related chronic visceral pain induced by CRD stimulation. Both EA and FCA interventions reversed these changes (Figure 4), indicating that EA can regulate the sensitization of neurons by inhibiting the activity of glial cells. Previous studies have demonstrated that glial cells in the spinal cord can maintain the sensitization of neurons to nociceptive stimuli, with P2X3 receptors playing a key role.18,32 The downregulatory effect of EA on the P2X3 receptor may be achieved by the inhibition of the activity of satellite glial cells. Activation of intestinal glial cells precedes that of intestinal neurons, suggesting that intestinal glial cells may be the initiating and maintaining factors in the pain response.33 The results of this study can partially explain this phenomenon, possibly because the inhibitory effect of EA on glial cell activity as a whole is mediated through the purine receptor family. Based on the mechanism underlying the neuro-glia interaction, the P2X7-P2Y1-P2X3 inhibition pathway34 is more suitable for explaining the analgesic mechanism of EA. This study indirectly proves that the excessive activation of EGCs is a crucial factor for the establishment and maintenance of chronic visceral pain in rats with IBS. Thus, as a non-noxious mechanical stimulus, acupuncture may reduce the sensitivity of the colon to noxious stimuli by activating a cell-level mediator, which may be glial cells. This is similar to the inhibitory effect of intermediate neurons on excitation-sensing neurons as described in the gate control theory. A limitation of this study is that there was no direct evidence to prove that acupuncture relieves the visceral pain of IBS through glial cells. To strengthen and validate our conclusions, further verification is warranted using additional experimental techniques such as the use of viruses or tools in mice and other methodologies, including photogenetics.
Funding Statement
Supported by National Basic Research Program of China: Research on the Initiation Mechanism of Moxibustion Effect and Its Endogenous Regulation Mechanism (973 program, No. 2015CB554501); National Natural Science Foundation of China: Interaction Mechanism of the Information between Electroacupuncture Stimulation to Zusanli and Visceral Pain in Dorsal Root Ganglion of Rats with Irritable Bowel Syndrome (No. 81873367); Study on the Mechanism of Periaqueductal gray Purinergic Ion Channel Receptor 3 Mediated in Electro-acupuncture Relieving Visceral Hypersensitivity in Mice with Irritable Bowel Syndrome (No. 81904301); Natural Science Foundation of Shanghai: based on Transient Receptor Potential Vanilloid 1 Mediated Calcium/Calmodulin-dependent Protein Kinase Ⅱ Signaling Pathway Involved in Electroacupuncture to Relieve Irritable Bowel Syndrome Mice Visceral Pain Mechanism Study (No. 22ZR1458600); Science and Technology Commission of Shanghai Municipality: Shanghai Clinical Research Center for Acupuncture and Moxibustion (No. 20MC1920500)
REFERENCES
- 1. Sinagra E, Morreale GC, Mohammadian G, et al. New therapeutic perspectives in irritable bowel syndrome: targeting low-grade inflammation, immuno-neuroendocrine axis, motility, secretion and beyond. World J Gastroenterol 2017; 23: 6593-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Császár-Nagy N, Bókkon I. Hypnotherapy and IBS: implicit, long-term stress memory in the ENS? Heliyon 2023; 9: e12751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grundy D. What activates visceral afferents? Gut 2004; 53: ii5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chao G, Wang Z, Zhang S. Research on correlation between psychological factors, mast cells, and PAR-2 signal pathway in irritable bowel syndrome. J Inflamm Res 2021; 14: 1427-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang F, Weng ZJ, Wu LY, et al. Etiology related irritable bowel syndrome animal models. Shi Jie Hua Ren Xiao Hua Za Zhi 2018; 26: 1772-77. [Google Scholar]
- 6. Barbara G, Cremon C, De Giorgio R, et al. Mechanisms underlying visceral hypersensitivity in irritable bowel syndrome. Curr Gastroenterol Rep 2011; 13: 308-15. [DOI] [PubMed] [Google Scholar]
- 7. Tian S, Zhang H, Chen S, Wu P, Chen M. Global research progress of visceral hypersensitivity and irritable bowel syndrome: bibliometrics and visualized analysis. Front Pharmacol 2023; 14: 1175057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morales-Soto W, Gulbransen BD. Enteric glia: a new player in abdominal pain. Cell Mol Gastroenterol Hepatol 2019; 7: 433-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhao M, Wang Z, Weng Z, et al. Electroacupuncture improves IBS visceral hypersensitivity by inhibiting the activation of astrocytes in the medial thalamus and anterior cingulate cortex. Evid Based Complement Alternat Med 2020; 2020: 2562979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Donnelly CR, Andriessen AS, Chen G, et al. Central nervous system targets: glial cell mechanisms in chronic pain. Neurotherapeutics 2020; 17: 846-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Seguella L, Gulbransen BD. Enteric glial biology, intercellular signalling and roles in gastrointestinal disease. Nat Rev Gastroenterol Hepatol 2021; 18: 571-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Soto F, Garcia-Guzman M, Stühmer W. Cloned ligand-gated channels activated by extracellular ATP (P2X receptors). J Membr Biol 1997; 160: 91-100. [DOI] [PubMed] [Google Scholar]
- 13. Fields RD, Burnstock G. Purinergic signalling in neuron-glia interactions. Nat Rev Neurosci 2006; 7: 423-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ciccarelli R, Di Iorio P, Ballerini P, et al. Effects of exogenous ATP and related analogues on the proliferat ionrate of dissociated primary cultures of rat astrocytes. J Neurosci Res 1994; 39: 556-66. [DOI] [PubMed] [Google Scholar]
- 15. Huang LZ, Ma JL, Wang YL, Xu X, Qin R, Wang J. The relation between krebs cycle of astrocytes and central sensitization in rats induced by formalin. Shen Jing Jie Pou Xue Za Zhi 2015; 3: 315-21. [Google Scholar]
- 16. Wu HG, Jiang B, Zhou EH, et al. Regulatory mechanism of electroacupuncture in irritable bowel syndrome: preventing MC activation and decreasing SP VIP secretion. Dig Dis Sci 2008; 53: 1644-51. [DOI] [PubMed] [Google Scholar]
- 17. Shi Y, Chen YH, Yin XJ, et al. Electroacupuncture versus moxibustion for irritable bowel syndrome: a randomized, parallel-controlled trial. Evid Based Complement Alternat Med 2015; 2015: 361786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weng ZJ, Wu LY, Zhou CL, et al. Effect of electroacupuncture on P2X3 receptor regulation in the peripheral and central nervous systems of rats with visceral pain caused by irritable bowel syndrome. Purinergic Signal 2015; 11: 321-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang F, Ma Z, Weng ZJ, et al. P2X3 receptor in primary afferent neurons mediates the relief of visceral hypersensitivity by electroacupuncture in an irritable bowel syndrome rat model. Gastroenterol Res Pract 2020; 2020: 8186106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. NC3Rs reporting guidelines working group. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 2010; 160: 1577-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic uisceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology 2000; 119: 1276-85. [DOI] [PubMed] [Google Scholar]
- 22. Weng ZJ, Wu LY, Lu Y, et al. Electroacupuncture diminishes P2X2 and P2X3 purinergic receptor expression in dorsal root ganglia of rats with visceral hypersensitivity. Neural Regen Res 2013; 8: 802-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fan F, Chen Y, Chen Z, et al. Blockade of BK channels attenuates chronic visceral hypersensitivity in an IBS-like rat model. Mol Pain 2021; 17: 17448069211040364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huang LY, Sun RR, Sun N, Zhou YF, Zeng F, Liang FR. Research progress of central-peripheral mechanism of acupuncture regulating visceral pain. Shi Jie Ke Xue Ji Shu-Zhong Yi Yao Xian Dai Hua 2021; 23: 232-8. [Google Scholar]
- 25. Sadeghi R, Heidarnia MA, Zagheri Tafreshi M, Rassouli M, Soori H. The reasons for using acupuncture for pain relief. Iran Red Crescent Med J 2014; 16: e15435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. He YX, Chu HR, Tong L, Li N, Sun PY, Wu LB. Effect of mild moxibustion on “Shangjuxu” and “Tianshu” points on the expression of orexin-A and its receptor in rats with irritable bowel syndrome. Liaoning Zhong Yi Yao Da Xue Xue Bao 2020; 22: 88-91. [Google Scholar]
- 27. Luo J, Ran N, Zhou JW, Hou XM. Analysis of acupoint selection rules for acupuncture treatment of diarrhea type irritable bowel syndrome based on data mining technology. Zhong Xi Yi Jie He Xin Xue Guan Bing Dian Zi Za Zhi 2020; 8: 156-7+160. [Google Scholar]
- 28. Weng ZJ, Hu SX, Zhang F, et al. Spinal cord astrocyte P2X7Rs mediate the inhibitory effect of electroacupuncture on visceral hypersensitivity of rat with irritable bowel syndrome. Purinergic Signal 2023; 19: 43-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ji RR, Xu ZZ, Gao YJ. Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discov 2014; 13: 533-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Greenwald JD, Shafritz KM. An Integrative neuroscience framework for the treatment of chronic pain: from cellular alterations to behavior. Front Integr Neurosci 2018; 12: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sun YN, Luo JY, Rao ZR, Lan L, Duan L. GFAP and Fos immunoreactivity in lumbo-sacral cord and medulla oblongata after chronic colonic inflammation in rats. World J Gastroenterol 2005; 11: 4827-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhou Y, Zhang F, Weng ZJ, et al. Regulation of GFAP and P2X3 receptors in the spinal cord of IBS rats with visceral pain. Shi Jie Ke Xue Ji Shu-Zhong Yi Yao Xian Dai Hua 2021; 23: 2919-27. [Google Scholar]
- 33. Allen NJ, Barres BA. Neuroscience: Glia - more than just brain glue. Nature 2009; 457: 675-7. [DOI] [PubMed] [Google Scholar]
- 34. Chen Y, Zhang X, Wang C, Li G, Gu Y, Huang LY. Activation of P2X7 receptors in glial satellite cells reduces pain through downregulation of P2X3 receptors in nociceptive neurons. Proc Natl Acad Sci USA 2008; 105: 16773-8. [DOI] [PMC free article] [PubMed] [Google Scholar]