Abstract
Background
Lazertinib is a potent, irreversible, third-generation epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) with significant efficacy in patients with EGFR T790M-mutated non-small cell lung cancer (NSCLC). This is the final overall survival (OS) report from the phase 1/2 LASER201 study in patients with advanced NSCLC with disease progression on or after prior EGFR TKI therapy.
Methods
Eligible patients were aged ≥ 20 years, with advanced EGFR-mutated NSCLC and previous therapy with EGFR TKI. Patients in this integrated analysis received oral lazertinib 240 mg/day. Endpoints included efficacy and safety; exploratory analyses included associations between circulating EGFR-mutant tumor DNA (ctDNA) and efficacy parameters.
Results
This integrated analysis included 78 patients in Korea who received second- or later-line lazertinib. The median OS was 38.9 months; estimated survival rates at 12, 24, and 36 months were 89.5%, 73.9%, and 52.8%, respectively. The cumulative 12-month incidence of central nervous system progression was 9.4%. EGFR-mutant ctDNA was detected in 46 patients (62.2%) at baseline. The presence of ctDNA at baseline significantly predicted progression-free survival (PFS), disease control rate (DCR), and OS. PFS, response rate, and DCR were significantly associated with EGFR-mutant ctDNA clearance at cycle 3; PFS and OS were significantly associated with ctDNA clearance at cycle 5. The safety profile of lazertinib 240 mg/day was consistent with previous findings.
Conclusions
Lazertinib is a promising treatment option for patients with EGFR T790M-positive NSCLC following disease progression on prior EGFR-directed TKIs. Patients in LASER201 experienced prolonged OS, regardless of their EGFR mutation, brain metastases, or prior brain radiation status. Clearance of plasma EGFR mutations after lazertinib was associated with patient outcomes.
Trial registration
ClinicalTrials.gov identifier NCT03046992.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12916-024-03620-8.
Keywords: ctDNA, Lazertinib, NSCLC, TKI, Overall survival
Background
The Thr790Met (T790M) gatekeeper mutation is the dominant mechanism of acquired resistance to first- and second-generation tyrosine kinase inhibitors (TKIs) in patients with advanced epidermal growth factor receptor (EGFR) mutation-positive non-small cell lung cancer (NSCLC) [1, 2]. Third-generation EGFR-directed TKIs were designed to overcome T790M-mediated resistance while retaining activity against common activating EGFR mutations (e.g., exon 19 deletion (Ex19Del), L858R). Osimertinib, a third-generation TKI, is standard of care for the treatment of patients with EGFR T790M-positive NSCLC [3, 4]. Other third-generation TKIs are either in late-stage clinical development or have been recently approved in Asia [5]. Beyond the TKIs, agents such as MET inhibitors and antibody–drug conjugates are currently under investigation, as are combinations of chemotherapy, immunotherapy, and antiangiogenic therapy [6–10]; clinical trials investigating these strategies are underway [10].
Lazertinib (YH25448, JNJ-73841937) is an oral, potent, irreversible, third-generation EGFR TKI. It has similar or greater inhibitory potency than osimertinib against mutated EGFR, including T790M, Ex19Del, and L858R, but less activity than osimertinib against wild-type EGFR [11]. Lazertinib demonstrated good blood–brain barrier penetration in a brain metastasis xenograft model [11].
In January 2021, lazertinib was approved in the Republic of Korea as a second-line treatment for patients with EGFR T790M-positive locally advanced or metastatic NSCLC previously treated with EGFR TKIs [12]. Approval was based on the phase 1/2 LASER201 study [13, 14], which showed that lazertinib was effective, had evidence of intracranial efficacy based on an independent central review, and had a manageable safety profile at the recommended therapeutic dose of 240 mg/day [15]. Median overall survival (OS) was not reached at the time of analysis (23.7% maturity) [15]. In June 2023, lazertinib was approved by the Ministry of Food and Drug Safety in the Republic of Korea as a first-line treatment for EGFR-mutated non-small cell lung cancer [12]. We now report an updated analysis of OS and safety of lazertinib 240 mg/day in patients with advanced EGFR T790M-positive NSCLC previously treated with EGFR TKIs from the LASER201 study, as well as the results of circulating tumor DNA (ctDNA) analyses, which were undertaken to identify potential associations between changes in detected EGFR mutations and patient outcomes.
Methods
Study design
LASER201 was a multicenter, open-label, phase 1/2 study conducted to evaluate the safety, tolerability, and efficacy of lazertinib in patients with locally advanced or metastatic EGFR-mutated NSCLC (ClinicalTrials.gov identifier: NCT03046992). The study methodology has been described previously [13]. This report focuses on a protocol-defined integrated analysis of patients who received the 240 mg dose of lazertinib as second- or later-line therapy.
ctDNA clearance analysis
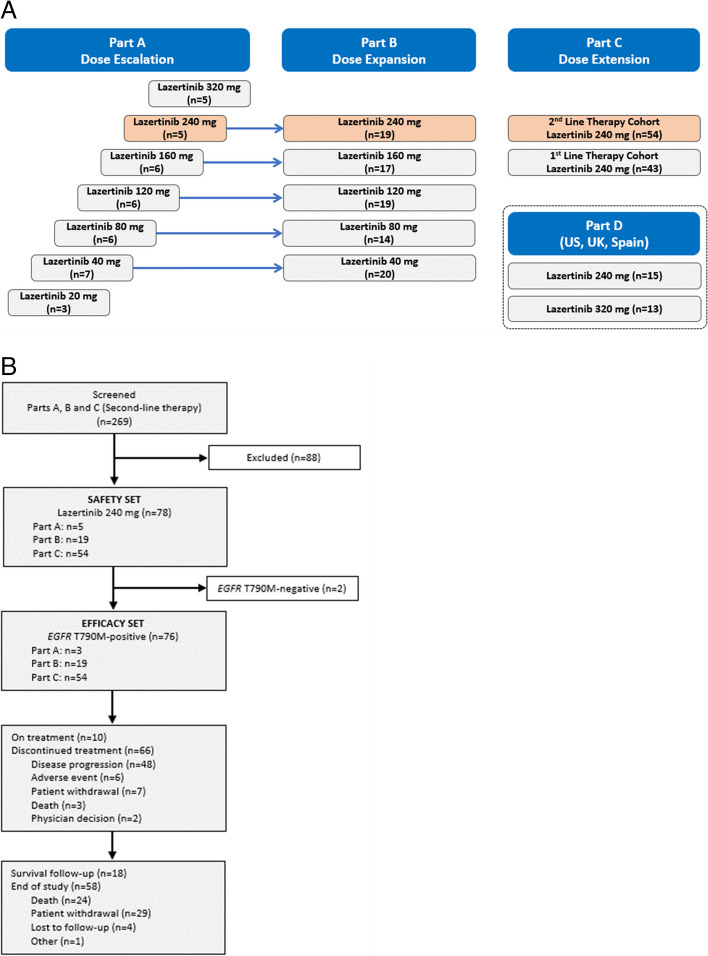
All patients in parts A, B, and C (the second-line cohort) of the study (Fig. 1A) provided mandatory plasma samples for analysis of ctDNA until the primary database lock at screening, and every two cycles thereafter until disease progression as per Response Evaluation Criteria in Solid Tumours (RECIST) version 1.1.
Fig. 1.
Study design (A) and patient disposition (B). Data cut-off: April 8, 2022. Orange shading in top panel shows the patient subgroup included in the present integrated analysis. EGFR epidermal growth factor receptor
Plasma samples of 10 ml were collected in EDTA tubes and centrifuged at 3000 × g for 10 min at 4 °C. Plasma was isolated within 4 h and stored at – 80 °C until the time of DNA extraction. Plasma cell-free DNA was extracted from 2 ml of the plasma using the QIAamp DSP Circulating NA Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions.
EGFR-mutated ctDNA analysis (Ex19Del/L858R/L861Q/G724S) was conducted in plasma samples with valid ctDNA results by droplet digital polymerase chain reaction (ddPCR) using a ddPCR system (QX200 Droplet Digital PCR System; Bio-Rad, Hercules, CA, USA) and EGFR mutation analysis kit (Droplex EGFR Mutation Test v2; Gencurix, Seoul, South Korea) as per the manufacturer’s recommended protocol. If EGFR mutations were no longer detectable, the sample was defined as “cleared.” If any of the EGFR mutations were still detectable, the sample was defined as “not cleared.” Detectability of EGFR-mutated ctDNA at baseline (cycle 1 day 1), and its clearance at weeks 6 (cycle 3 day 1) and 12 (cycle 5 day 1)—specifically in patients with detectable EGFR-mutated ctDNA at baseline—were analyzed in relation to progression-free survival (PFS) per independent central review (according to RECIST version 1.1), OS, objective response rate (ORR), and disease control rate (DCR).
Recognizing the clinical significance of the T790M mutation, particularly in the context of resistance to EGFR-targeted therapy, we conducted an additional supplementary analysis to incorporate mutation status using the Guardant 360 test [14]. This analysis aimed to provide further insights into the genetic landscape of patients undergoing lazertinib treatment and its potential implications for treatment response and resistance mechanisms.
Statistical analysis
An integrated analysis of patients receiving lazertinib from parts A, B, and C of the study was performed per protocol. The data cut-off dates for PFS and OS assessments were January 8, 2021, and April 8, 2022, respectively. The data cut-off date for safety assessment was March 30, 2023. The safety set included all patients who received at least one dose of lazertinib. Safety data were analyzed by descriptive statistical methods.
The efficacy set included patients in the safety set who had baseline RECIST (version 1.1) assessment and whose tumor EGFR mutation status was centrally confirmed. Kaplan–Meier methods were used to evaluate OS and to estimate the proportion of patients without an event, with 95% confidence intervals (CIs), at 12, 24, and 36 months.
Exploratory analyses included: subgroup analyses of OS according to baseline characteristics; time to first subsequent therapy or death and time to second subsequent therapy or death, which were defined as the interval from the first multiple-dose date (cycle 1 day 1) to start date of first/second subsequent anticancer therapy (excluding radiotherapy) following study treatment discontinuation or death, whichever was earlier. All analyses were performed using SAS (version 9.4; SAS Institute Inc., Cary, NC, USA).
Cell-free DNA next-generation sequencing was conducted by Guardant Health, Inc. (Redwood City, CA) using Guardant 360, as previously described [14]. The baseline genetic alteration status determined by the Guardant 360 test was further analyzed according to ctDNA cleared or not cleared at cycle 3, which was confirmed by ddPCR and tumor shrinkage.
Results
Patient population
Between February 15, 2017, and May 10, 2019, 224 patients were enrolled in study parts A, B, and C (Fig. 1A). For the present integrated analysis, 78 patients received lazertinib 240 mg/day as second- or later-line therapy and were included in the safety set. All patients were Asian and had received at least one prior EGFR TKI (Table 1). The median duration of lazertinib treatment was 13.3 months (range, 0.3–50.3 months; Additional file 1: Table S1). Seventy-six patients had EGFR T790M-positive disease by central testing at baseline and constituted the efficacy set. At data cut-off (April 8, 2022), 10 of 76 patients remained on treatment and 66 had discontinued treatment, primarily because of disease progression (Fig. 1B).
Table 1.
Baseline demographics and disease characteristics (safety set)
| Characteristic | Lazertinib 240 mg (n = 78) |
|---|---|
| Age, median (range), years | 62 (33–82) |
| Race, n (%) | |
| Asian | 78 (100.0) |
| Sex, n (%) | |
| Male | 40 (51.3) |
| Female | 38 (48.7) |
| ECOG performance status, n (%) | |
| 0 | 20 (25.6) |
| 1 | 58 (74.4) |
| Tumor histology, n (%) | |
| Adenocarcinoma | 74 (94.9) |
| Adeno-squamous carcinoma | 2 (2.6) |
| Other | 2 (2.6) |
| Stage (AJCC 7th edition), n (%) | |
| III | 3 (3.8) |
| IV | 75 (96.2) |
| Brain metastases at baseline (investigator assessment), n (%) | 40 (51.3) |
| EGFR mutation status (central testing), n (%) | |
| Exon19Del | 53 (67.9) |
| L858R | 23 (29.5) |
| L861Q | 1 (1.3) |
| Negative (T790M-positive only) | 1 (1.3) |
| T790M status (central testing), n (%) | |
| Positive | 76 (97.4) |
| Negative | 2 (2.6) |
| Prior lines of systemic therapy, n (%) | |
| 1 | 50 (64.1) |
| ≥ 2 | 28 (35.9) |
| Number of prior EGFR TKIs, median (range) | 1 (1–3) |
| Prior EGFR TKI,a n (%) | 78 (100.0) |
| Gefitinib | 40 (51.3) |
| Afatinib | 28 (35.9) |
| Erlotinib | 16 (20.5) |
Note: Some totals do not add up to 100% due to rounding
AJCC American Joint Committee on Cancer, EGFR epidermal growth factor receptor, ECOG Eastern Cooperative Oncology Group, TKI tyrosine kinase inhibitor
aPatients may have received more than one prior EGFR TKI
Overall survival
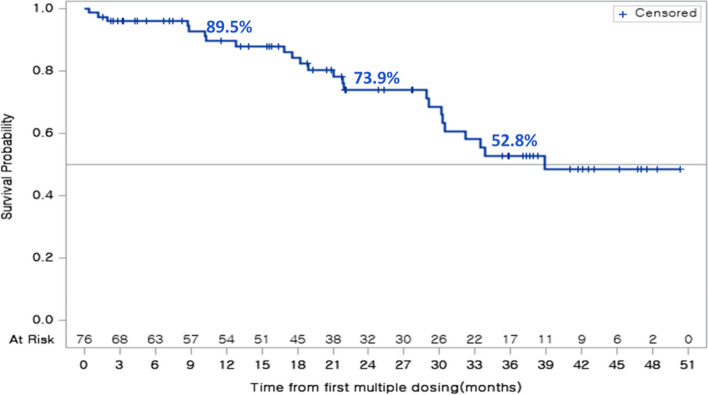
The median duration of follow-up for OS was 27.7 months (interquartile range, 15.4–41.7 months); 24 of 76 patients (31.6%) had died at the time of analysis. Median OS was 38.9 months (95% CI 30.2–not reached [NR]) (Fig. 2). Survival rates at 12, 24, and 36 months were 89.5% (95% CI 79.2–94.9), 73.9% (95% CI 60.1–83.5), and 52.8% (95% CI 37.0–66.3), respectively.
Fig. 2.
Overall survival in patients with EGFR T790M-positive tumors (efficacy set). Data cut-off: April 8, 2022. CI confidence interval, EGFR epidermal growth factor receptor, NR not reached
Subgroup analyses
Subgroup analyses of OS by baseline characteristics are presented in Additional file 2: Fig. S1. When analyzed by EGFR mutation type at baseline, median OS was NR (95% CI 30.5–NR) in patients harboring an Ex19Del (n = 53) and 29.1 months (95% CI 10.3–NR) in patients with L858R mutations (n = 22) (Additional file 2: Fig. S1A).
When analyzed by the presence or absence of brain metastases at baseline, median OS was NR (95% CI 21.1–NR) in patients with brain metastases (n = 25) and 38.9 months (95% CI 30.1–NR) in patients without brain metastases (n = 51) (Additional file 2: Fig. S1B).
Lazertinib showed consistent efficacy regardless of brain metastasis status (Additional file 2: Fig. S1B) and prior brain radiation therapy status (Additional file 2: Fig. S1C). For patients with baseline central nervous system (CNS) metastases, the cumulative incidence rate of CNS progression at 12 months was 9.4% (Additional file 2: Fig. S2).
Time to first or second subsequent therapy or death
Thirteen (17.1%) and 15 (19.7%) of the 76 patients received osimertinib and a platinum doublet, respectively, as subsequent therapy. Kaplan–Meier curves for time to first or second subsequent therapy or death in patients with EGFR T790M-positive tumors are presented in Additional file 2: Fig. S3. Median time to first subsequent therapy or death was 21.0 months (95% CI 13.8–32.2). Median time to second subsequent therapy or death was 29.1 months (95% CI 22.0–38.9).
Safety
In the safety set of patients who received lazertinib 240 mg/day (n = 78), 76 (97.4%) reported at least one treatment-emergent adverse event (Table 2). Treatment-emergent adverse events were considered to be drug-related by the investigator in 69 patients (88.5%). The most common drug-related treatment-emergent adverse events of any grade were rash (n = 29; 37.2%), paresthesia (n = 26; 33.3%), pruritus (n = 25; 32.1%), and muscle spasms (n = 21; 26.9%) (Additional file 1: Table S2). Drug-related grade 3 treatment-emergent adverse events were reported in 11 patients (14.1%). No drug-related grade 4 or 5 events were reported.
Table 2.
Summary of treatment-emergent adverse events (safety set; n = 78)
| Parameter | No. of patients (%) | |
|---|---|---|
| TEAE | 76 (97.4) | |
| Drug-related TEAE | 69 (88.5) | |
| Serious TEAE | 21 (26.9) | |
| Drug-related serious TEAE | 3 (3.8) | |
| Grade ≥ 3 TEAE | 28 (35.9) | |
| Drug-related grade ≥ 3 TEAE | 11 (14.1) | |
| TEAE leading to death | 3 (3.8) | |
| Drug-related TEAE leading to death | 0 | |
| TEAE leading to dose reduction | 13 (16.7) | |
| TEAE leading to temporary drug interruption | 17 (21.8) | |
| TEAE leading to permanent drug withdrawal | 6 (7.7) | |
| TEAEs occurring in ≥ 10% of patients | All grades | Grade ≥ 3 |
| Rash | 29 (37.2) | 1 (1.3) |
| Paresthesia | 28 (35.9) | 2 (2.6) |
| Pruritus | 27 (34.6) | 0 |
| Headache | 22 (28.2) | 0 |
| Muscle spasms | 22 (28.2) | 0 |
| Diarrhea | 21 (26.9) | 1 (1.3) |
| Decreased appetite | 20 (25.6) | 0 |
| Cough | 16 (20.5) | 0 |
| Paronychia | 16 (20.5) | 1 (1.3) |
| Constipation | 15 (19.2) | 0 |
| Nausea | 13 (16.7) | 0 |
| Fatigue | 12 (15.4) | 0 |
| Aspartate aminotransferase increased | 11 (14.1) | 0 |
| Alanine aminotransferase increased | 10 (12.8) | 0 |
| Myalgia | 10 (12.8) | 0 |
| Pulmonary embolism | 10 (12.8) | 5 (6.4) |
| Dizziness | 10 (12.8) | 0 |
| Dyspepsia | 9 (11.5) | 0 |
| Stomatitis | 9 (11.5) | 0 |
| Blood creatinine increased | 9 (11.5) | 0 |
| Vomiting | 8 (10.3) | 1 (1.3) |
| Dry skin | 8 (10.3) | 0 |
Note: Data cut-off: March 30, 2023. Patients with two or more adverse events with the same term were counted only once for that adverse-event term
TEAE treatment-emergent adverse event
Serious adverse events were reported in 21 patients (26.9%); these were considered drug-related in three patients (3.8%), one with gastritis, one with pneumonia, and one with pneumonitis. Drug-related QT interval prolongation (grade 1) was reported in three patients (3.8%).
Dose reductions, interruptions, and discontinuation because of adverse events were required in 13 (16.7%), 17 (21.8%), and 6 (7.7%) patients who received lazertinib 240 mg/day, respectively. Adverse events leading to drug discontinuation were pneumonitis (n = 1), pulmonary embolism (n = 1), peripheral neuropathy (n = 1), herpes zoster and aspiration pneumonia (n = 1), cerebellar infarction (n = 1), and drug intoxication due to pesticide (n = 1).
ctDNA clearance analysis
Among the 74 patients who received lazertinib 240 mg/day and who had a sample for ctDNA analysis, EGFR-mutated ctDNA was detected in 46 patients (62.2%) at baseline and not detected in 27 patients (36.5%); one patient had an invalid result (Additional file 2: Fig. S4). Among patients who had cleared ctDNA by cycles 3 and 5, seven patients had continued treatment for longer than 2 years (21.2% and 22.6%, respectively); all patients who did not have ctDNA clearance had a treatment duration of less than 2 years (Additional file 1: Table S1).
Overall, the “cleared” group showed a better prognostic trend in terms of key anti-tumor efficacy endpoints, including ORR, DCR, PFS, and OS, compared with the “not cleared” group at baseline, cycle 3, and cycle 5. In particular, significant prognostic superiority was observed for PFS, ORR, and DCR at cycle 3 (Table 3; Additional file 2: Fig. S5).
Table 3.
Analysis of non-clearance or clearance of plasma EGFR mutations
| Variable | Baseline ctDNA | Cycle 3 ctDNA clearance | Cycle 5 ctDNA clearance | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
Yes (n = 46) |
No (n = 27) |
P-value |
Cleared (n = 33) |
Not cleared (n = 11) |
P-value |
Cleared (n = 31) |
Not cleared (n = 5) |
P-value | |
| ORRa, n (%) | 24 (52.2) | 16 (59.3) | 0.5571 | 21 (63.6) | 3 (27.3) | 0.0359 | 22 (71.0) | 2 (40.0) | 0.3074 |
| DCRb, n (%) | 38 (82.6) | 27 (100.0) | 0.0226 | 32 (97.0) | 6 (54.5) | 0.0022 | 30 (96.8) | 4 (80.0) | 0.2619 |
| Median PFSc, months [95% CI]d | 5.6 [4.1–11.1] | 23.3 [15.0–NR] | 0.0002e | 11.1 [5.4–13.6] | 5.4 [1.3–8.2] | 0.0012e | 11.0 [5.5–13.6] | 4.0 [1.3–NR] | 0.0037e |
| Hazard ratio [95% CI] | 0.30 [0.15–0.59] | 0.0004 | 0.28 [0.12–0.64] | 0.0026 | 0.0087 | ||||
| Median OSf, months [95% CI]d | 32.2 [21.8–NR] | NR [30.5–NR] | 0.0235e | 33.4 [21.1–NR] | 29.6 [28.9–NR] | 0.8447e | 33.4 [21.8–NR] | NR [8.8–NR] | 0.0151e |
| Hazard ratio [95% CI] | 0.35 [0.14–0.90] | 0.86 [0.19–3.95] | |||||||
Note: Data cut-off: January 8, 2021 (PFS), and April 8, 2022 (OS). Percentages are calculated based on number of evaluable patients
aORR is the proportion of patients with a confirmed best overall response of CR or PR
bDCR is the proportion of patients who have a best overall response of CR or PR or SD (SD at ≥ 5 weeks)
cPFS is measured from the first multiple-dose date (cycle 1 day 1) until objective tumor progression or death, whichever occurs first
dMedian and 95% CI are calculated using Kaplan–Meier estimates
eLog-rank test
fOS is measured from the first multiple-dose date (cycle 1 day 1) until death due to any cause or date of last known alive
CI confidence interval, CR complete response, ctDNA circulating tumor DNA, DCR disease-control rate, NR not reached, ORR objective response rate, OS overall survival, PFS progression-free survival, PR partial response, SD stable disease
Genetic mutations related to the mitogen-activated protein kinase (MAPK), phosphoinositide 3-kinase (PI3K), and cyclic adenosine monophosphate signaling pathways showed a tendency to be more frequently detected at baseline in the not cleared group compared with the cleared group. This suggests that components in these pathways may be associated with lazertinib resistance mechanisms (Additional file 2: Fig. S6).
Among patients in the cleared group (n = 21), baseline genetic alterations related to cell cycle and DNA repair were observed relatively more frequently in patients with significant tumor shrinkage (≥ 50%) than in those with shrinkage of less than 10%. In the not cleared group, genetic alterations in BRAF and MAPK1 in the MAPK signaling pathway were more frequent in patients with less tumor shrinkage at cycle 3 (Additional file 2: Fig. S7).
Discussion
The first reported analysis of the LASER201 study established the efficacy and safety of lazertinib 240 mg/day in patients with advanced EGFR T790M-positive NSCLC who had progressed on prior therapy with EGFR-directed TKIs [14]. OS data were not mature at that time. We now report updated findings from the same integrated analysis after a median follow-up of 27.7 months. At this time, the median OS with lazertinib 240 mg/day was 38.9 months (95% CI 30.2–NR), with an estimated survival rate of 52.8% at 36 months. These findings compare favorably with those reported for osimertinib from a pooled analysis of the AURA extension and AURA2 studies (median OS, 26.8 months; 36-month survival rate, 37%) and from the phase 3 AURA3 study (median OS, 26.8 months; 36-month survival rate, 30%) in a similar patient population [16, 17]. Median times to first subsequent therapy and second subsequent therapy or death with lazertinib in LASER201 (21.0 and 29.1 months, respectively) were also consistent with the AURA3 findings for osimertinib (16.0 and 20.0 months, respectively) [17].
The median OS was relatively longer in patients harboring an Ex19Del (NR; 95% CI 30.5–NR) in patients with L858R mutations (29.1 months; 95% CI 10.3–NR). This observation is aligned with multiple reports suggesting that outcomes with EGFR TKIs can be influenced by EGFR mutation type. For example, median OS following treatment with osimertinib was 29.1 months in patients with EGFR T790M-positive NSCLC with Ex19Del versus 21.4 months in patients with L858R mutations [16].
We investigated the dynamics of EGFR mutations in ctDNA to predict outcomes in lazertinib-treated patients in LASER201. Patients who were identified as ctDNA cleared at cycle 3 had better outcomes in the key anti-tumor efficacy endpoints of PFS, ORR, and DCR than those in the not cleared group. Numerous studies have explored the association of ctDNA clearance with clinical outcomes for first-, second-, and third-generation TKIs [18–20]. Recently, much effort has been invested in the evaluation and validation of ctDNA as a prognostic biomarker to guide clinical conversations around expected treatment outcomes across various advanced cancers, including NSCLC, and colorectal, breast, and pancreatic cancers [21–23]. Our data provide additional evidence for this approach, although further confirmatory studies are needed.
Several factors likely contributed to the favorable outcomes observed in LASER201. Lazertinib had a high clearance rate for EGFR mutations, with ctDNA clearance in 33 of 46 patients (71.7%) at cycle 3 and in 31 of 46 patients (67.4%) after cycle 5. In AURA3, 83 of 189 patients (43.9%) with baseline EGFR mutations and evaluable plasma samples at week 6 had detectable EGFR mutations [24]. The intracranial efficacy of lazertinib, evidenced by the median intracranial PFS of 26.0 months [14], may also contribute to the observed favorable OS. Furthermore, lazertinib showed consistent efficacy regardless of brain metastasis status and prior brain radiation therapy. For patients with baseline CNS metastases, the cumulative incidence rate of CNS progression at 12 months was 9.4%, which is lower than the 16% previously reported in AURA3 [25], although cross-trial comparisons are made with caution.
The updated safety analysis was consistent with earlier reports [13, 14] and supports the long-term safety of lazertinib 240 mg/day, with no new safety signals. Median time on treatment was more than 1 year (13 months), with some patients receiving lazertinib for up to 4 years. The most common drug-related adverse events with lazertinib 240 mg/day were skin toxicities (rash, pruritus) and paresthesia, which were typically mild or moderate in severity. The risk of cardiac events with lazertinib 240 mg/day, including corrected QT interval prolongation or reduced left ventricular ejection fraction, was low after extended follow-up. These findings align with a detailed cardiac safety assessment by Jang and colleagues, which suggests that lazertinib is not associated with an increased cardiac risk and has a small predicted magnitude of Fridericia’s corrected QT interval increase (2.2 ms) at maximum steady-state plasma concentration when dosed at 240 mg/day [26].
This was a single-arm study with a small sample size in a Korean patient population. Two ongoing international phase 3 trials involving lazertinib in patients with metastatic EGFR-mutated NSCLC are currently in progress. LASER301 compared lazertinib with gefitinib as first-line therapy in advanced EGFR-mutated NSCLC (ClinicalTrials.gov identifier: NCT04248829) [15]. Median PFS was statistically significantly longer with lazertinib versus gefitinib (20.6 vs 9.7 months; HR 0.45; 95% CI 0.34–0.58; p < 0.001). A similar result was observed in the subpopulation of Asian patients [27]. MARIPOSA, a study comparing lazertinib plus amivantamab versus osimertinib as first-line treatment in advanced EGFR-mutated NSCLC, included lazertinib monotherapy to assess the contribution of components (Clinicaltrials.gov identifier NCT04487080). With a median follow-up of 22 months, the median PFS was numerically longer with lazertinib (18.5 months; 95% CI 14.8–20.1) compared with osimertinib (16.6 months; 95% CI 14.8–18.5) [28].
Some limitations of this study warrant consideration. The proportion of patients with invalid results increased as treatment progressed, which may have been a result of treatment interventions impacting on the quality and quantity of ctDNA that was collected as previously reported [29]. There was an incomplete pairing of ctDNA and Guardant 360 results, as not all patients had ctDNA and Guardant 360 results, thus limiting comprehensive paired analysis. A more in-depth assessment of treatment response and mutation status would have been possible if matched ctDNA and Guardant 360 results had been available for each patient. In total, 29 analysis sets included both ctDNA analysis and Guardant 360 results, so interpretation of these results must be approached cautiously.
Interpretation of results such as these requires careful consideration because of the heterogeneity in patients’ response to treatment, which is attributed to inherent biological differences, disease characteristics, and other unidentified factors.
Conclusions
In summary, lazertinib is a promising treatment option for patients with EGFR T790M-positive NSCLC following EGFR-directed TKIs. Patients experienced prolonged OS, regardless of brain metastases or prior brain radiation, with a median OS of over 3 years. The safety profile of lazertinib 240 mg/day was consistent with previous findings and supportive of the long-term safety of lazertinib in this patient population. The ctDNA analyses suggest that clearance of plasma EGFR mutations has the potential to predict clinical outcomes in patients with EGFR-mutated NSCLC, although further confirmatory studies are needed.
Supplementary Information
Additional file 1: Table S1. Duration of lazertinib treatment in ctDNA Cleared group and Not Cleared group. Table S2. Drug-related treatment-emergent adverse events occurring in ≥ 10% of patients. Table S3. Ethics committee approvals.
Additional file 2: Fig. S1. Subgroup analyses of overall survival by I mutation type, brain metastases, and brain radiotherapy. Fig. S2. Cumulative incidence rate of CNS progression in lazertinib-treated patients in the LASER201 trial. Fig. S3. Time to first or second subsequent therapy or death in patients with EGFR T790M-positive non-small cell lung cancer. Data cut-off April 8, 2022. Fig. S4. Patient disposition for ctDNA analysis. Patients were categorized based on their clearance status as determined by ddPCR. Fig. S5. Analysis of plasma EGFR mutations at baseline, cycle 3, and cycle 5 in patients with a valid ctDNA sample at baseline and cycle 3 and/or cycle 5. Kaplan–Meier estimates showing OS and independent central review-assessed PFS according to the presence of ctDNA at baseline, cycle 3, and cycle 5. Fig. S6. Resistance mechanism with ctDNA clearance. Fig. S7. Summary of baseline genetic alterations at screening by ctDNA clearance at cycle 3 or not.
Acknowledgements
The authors thank the patients and their families, and the staff and investigators at all study sites. Medical writing and editorial support were provided by Lee Miller, BSc (Hons), and Harriet Lamb, BSc (Hons), of Miller Medical Communications Ltd., UK, and was funded by Yuhan Corporation. Yuhan Corporation was responsible for the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript; and the decision to submit the manuscript for publication.
Abbreviations
- CI
Confidence interval
- CNS
Central nervous system
- ctDNA
Circulating tumor DNA
- DCR
Disease-control rate
- ddPCR
Droplet digital polymerase chain reaction
- EGFR
Epidermal growth factor receptor
- Ex19Del
Exon 19 deletion
- MAPK
Mitogen-activated protein kinase
- NR
Not reached
- NSCLC
Non-small cell lung cancer
- ORR
Objective response rate
- OS
Overall survival
- PCR
Polymerase chain reaction
- PFS
Progression-free survival
- PI3K
Phosphoinositide 3-kinase
- RECIST
Response Evaluation Criteria in Solid Tumours
- TKI
Tyrosine kinase inhibitor
Authors’ contributions
J-YH: conceptualization, investigation, methodology, data curation, writing—review and editing. BCC: data curation, resources, investigation, writing—review and editing. M-JA: data curation, resources, investigation, writing—review and editing. KHL: data curation, resources, investigation, writing—review and editing. Y-GL: data curation, resources, investigation, writing—review and editing. D-WK: data curation, resources, investigation, writing—review and editing. YJM: data curation, resources, investigation, writing—review and editing. S-WK: data curation, resources, investigation, writing—review and editing. EKC: data curation, resources, investigation, writing—review and editing. J-HK: data curation, resources, investigation, writing—review and editing. G-WL: data curation, resources, investigation, writing—review and editing. SSL: data curation, resources, investigation, writing—review and editing. NML: data curation, software, validation, formal analysis, visualization, writing—review and editing. HH: data curation, software, validation, formal analysis, visualization, writing—review and editing. HWJ: resources; writing—review and editing. HP: visualization, writing—original draft, writing—review and editing. JL: visualization, writing—original draft, writing—review and editing. All authors read and approved the final manuscript.
Funding
LASER201 Parts A, B, and C were sponsored by Yuhan Corporation and Korea Drug Development Fund funded by the Ministry of Science and ICT, Ministry of Trade, Industry, and Energy, and Ministry of Health and Welfare (KDDF-201803–05, Republic of Korea).
Availability of data and materials
Deidentified participant data will be made available when all endpoints have been evaluated. Any requests for trial data and supporting material (data dictionary and statistical analysis plan) will be reviewed by the trial management group in the first instance. Only requests that have a methodologically sound proposal and whose proposed use of the data has been approved by the independent trial steering committee will be considered. Proposals should be directed to the corresponding author in the first instance; to gain access, data requestors will need to sign a data access agreement.
Declarations
Ethics approval and consent to participate
The study was performed in accordance with the Declaration of Helsinki and International Conference on Harmonization Good Clinical Practice guidelines. The protocol and any amendments were approved by institutional review boards at participating centers (Additional file 1: Table S3). Patients provided written informed consent before study participation.
Consent for publication
Not applicable.
Competing interests
Ji-Youn Han reports: grants or contracts from Takeda, Ono, Roche, and Pfizer; consulting fees from AstraZeneca, Takeda, Amgen, Janssen, Merck, Novartis, J INTS Bio, Oncobix, and Daiichi Sancho; payments or honoraria for lectures, presentations, speakers’ bureaux, or writing from AstraZeneca, Takeda, Janssen, Pfizer, Merck, Novartis, and Yuhan; payment for expert testimony from AstraZeneca; payment for participating in data safety monitoring boards or advisory boards for AstraZeneca, Janssen, J INTS Bio, and Abion; leadership or fiduciary role in Health Insurance Review and Assessment.
Byoung Chul Cho reports: research funding from MOGAM Institute, LG Chem, Oscotec, Interpark Bio Convergence Corp, GIInnovation, GI-Cell, Abion, Abbvie, AstraZeneca, Bayer, Blueprint Medicines, Boehringer Ingelheim, Champions Oncology, CJ Bioscience, CJ Blossom Park, Cyrus, Dizal Pharma, Genexine, Janssen, Lilly, MSD, Novartis, Nuvalent, Oncternal, Ono, Regeneron, Dong-A ST, Bridgebio therapeutics, Yuhan, ImmuneOncia, Illumina, Kanaph Therapeutics, Therapex, J INT Sbio, Hanmi, CHA Bundang Medical Center, and Vertical Bio AG; royalties from Champions Oncology, Crown Bioscience, Imagen, and Pearl River Bio GmbH; consulting fees from Abion, BeiGene, Novartis, AstraZeneca, Boehringer-Ingelheim, Roche, BMS, CJ, CureLogen, Cyrus Therapeutics, Ono, Onegene Biotechnology, Yuhan, Pfizer, Eli Lilly, GI-Cell, Guardant, HK Inno-N, Imnewrun Biosciences Inc., Janssen, Takeda, MSD, Janssen, Medpacto, Blueprint Medicines, and RandBio, Hanmi; payments for presentations from ASCO, AstraZeneca, Guardant, Roche, ESMO, IASLC, Korean Cancer Association, Korean Society of Medical Oncology, Korean Society of Thyroid-Head and Neck Surgery, Korean Cancer Study Group, Novartis, MSD, The Chinese Thoracic Oncology Society, Pfizer; payments for advisory boards from KANAPH Therapeutic Inc, BridgeBio, Cyrus Therapeutics, Guardant Health, Oscotec Inc, J INTS BIO, Therapex Co., Ltd, Gliead, and Amgen; membership of the board of directors of J INTS BIO; stock ownership in TheraCanVac Inc, Gencurix Inc, BridgeBio, KANAPH Therapeutic Inc, Cyrus Therapeutics, Interpark Bio Convergence Corp., and J INTS BIO; employee of Yonsei University Health System; and founder of DAAN Biotherapeutics.
Myung-Ju Ahn reports: consulting fees from AstraZeneca, Roche, MSD, Merck, Takeda, ONO, Novartis, Lilly, Amgen, Yuhan Corporation, and Alpha Pharmaceuticals; and payments or honoraria for lectures, presentations, speakers’ bureaux, or writing from AstraZeneca, Roche, MSD, Merck, Takeda, Ono, Novartis, Lilly, Amgen, and Yuhan Corporation.
Ki Hyeong Lee reports: grants or contracts from Merck Serono; and consulting fees from MSD, Pfizer, Eli Lilly, Yuhan, AstraZeneca, and BMS.
Yun-Gyoo Lee reports: payments or honoraria for lectures, presentations, speakers’ bureaux, or writing from AstraZeneca, MSD, Yuhan, Lilly, and Boehringer Ingelheim; payment for participating in data safety monitoring boards or advisory boards for BeiGene, Takeda, Guardant Health, Yuhan Corporation, Ono, and Novartis.
Dong-Wan Kim reports: clinical trial research funding (to institution) from Alpha Biopharma, Amgen, AstraZeneca, Boehringer-Ingelheim, BridgeBio, BMS, Chong Keun Dang, Daiichi-Sankyo, GSK, Hanmi, Janssen, Merck, Meurs, Mirati, MSD, Novartis, ONO Pharmaceutical, Pfizer, Roche/Genentech, Takeda, TP Therapeutics, Xcovery; laboratory research funding (to institution) from InnoN; medical writing assistance from Amgen, AstraZeneca, Boehringer-Ingelheim, BridgeBio, BMS, Chong Keun Dang, Daiichi-Sankyo, GSK, Pfizer, MSD, Merck, Merus, Novartis, Roche, Takeda; honoraria from the Korean Association for Lung Cancer, Korean Cancer Association, Korean Society of Medical Oncology, Taiwan Lung Cancer Society, Asian Thoracic Oncology Research Group; travel support for attending meetings from the International Association for the Study of Lung Cancer, Asian Thoracic Oncology Research Group, and the Taiwan Lung Cancer Society; unpaid participation on advisory boards for Amgen, AstraZeneca, BMS/ONO Pharmaceuticals, Daiichi-Sankyo, GSK, Janssen, Meck, MSD, Pfizer, SK Biopharm, and Takeda; member of board of directors for the Asian Thoracic Oncology Research Group, Korean Association for Lung Cancer, Korean Cancer Association, Korean Society of Medical Oncology; and paid scientific advisor for the Health Insurance Review and Assessment Service, Republic of Korea.
Young Joo Min reports no conflicts.
Sang-We Kim reports no conflicts.
Eun Kyung Cho reports participation in a data safety monitoring meeting for the Yuhan Corporation for the present study and receipt of study drug from the Yuhan Corporation for the present study.
Joo-Hang Kim reports no conflicts.
Gyeong-Won Lee reports no conflicts.
Sung Sook Lee reports no conflicts.
Na Mi Lee is employed by the Yuhan Corporation.
Hyun Woo Jang is employed by the Yuhan Corporation.
Heewon Han is employed by the Yuhan Corporation.
Hyejoo Park is employed by the Yuhan Corporation.
Jieon Lee is employed by the Yuhan Corporation.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Oxnard GR, Arcila ME, Sima CS, Riely GJ, Chmielecki J, Kris MG, Pao W, Ladanyi M, Miller VA. Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR-mutant lung cancer: distinct natural history of patients with tumors harboring the T790M mutation. Clin Cancer Res. 2011;17(6):1616–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu HA, Arcila ME, Rekhtman N, Sima CS, Zakowski MF, Pao W, Kris MG, Miller VA, Ladanyi M, Riely GJ. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013;19(8):2240–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, Mok TS, Reck M, Van Schil PE, Hellmann MD, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv192–237. [DOI] [PubMed] [Google Scholar]
- 4.Wu YL, Planchard D, Lu S, Sun H, Yamamoto N, Kim DW, Tan DSW, Yang JC, Azrif M, Mitsudomi T, et al. Pan-Asian adapted Clinical Practice Guidelines for the management of patients with metastatic non-small-cell lung cancer: a CSCO-ESMO initiative endorsed by JSMO, KSMO, MOS, SSO and TOS. Ann Oncol. 2019;30(2):171–210. [DOI] [PubMed] [Google Scholar]
- 5.Lau SCM, Ou SI. And still they come over troubled waters: Can Asia’s third-generation EGFR tyrosine kinase inhibitors (furmonertinib, aumolertinib, rezivertinib, limertinib, befotertinib, SH-1028, and lazertinib) affect global treatment of EGFR+ NSCLC. J Thorac Oncol. 2022;17(10):1144–54. [DOI] [PubMed] [Google Scholar]
- 6.Mollica V, Rizzo A, Marchetti A, Tateo V, Tassinari E, Rosellini M, Massafra R, Santoni M, Massari F. The impact of ECOG performance status on efficacy of immunotherapy and immune-based combinations in cancer patients: the MOUSEION-06 study. Clin Exp Med. 2023;23(8):5039–49. [DOI] [PubMed] [Google Scholar]
- 7.Rizzo A. Identifying optimal first-line treatment for advanced non-small cell lung carcinoma with high PD-L1 expression: a matter of debate. Br J Cancer. 2022;127(8):1381–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rizzo A, Cusmai A, Giovannelli F, Acquafredda S, Rinaldi L, Misino A, Montagna ES, Ungaro V, Lorusso M, Palmiotti G. Impact of proton pump inhibitors and histamine-2-receptor antagonists on non-small cell lung cancer immunotherapy: A systematic review and meta-analysis. Cancers (Basel). 2022;14(6):1404. [DOI] [PMC free article] [PubMed]
- 9.Santoni M, Rizzo A, Mollica V, Matrana MR, Rosellini M, Faloppi L, Marchetti A, Battelli N, Massari F. The impact of gender on The efficacy of immune checkpoint inhibitors in cancer patients: The MOUSEION-01 study. Crit Rev Oncol Hematol. 2022;170: 103596. [DOI] [PubMed] [Google Scholar]
- 10.Meng Y, Bai R, Cui J. Precision targeted therapy for EGFR mutation-positive NSCLC: Dilemmas and coping strategies. Thorac cancer. 2023;14(13):1121–34. [DOI] [PMC free article] [PubMed]
- 11.Yun J, Hong MH, Kim SY, Park CW, Kim S, Yun MR, Kang HN, Pyo KH, Lee SS, Koh JS, et al. YH25448, an irreversible EGFR-TKI with potent intracranial activity in EGFR mutant non-small cell lung cancer. Clin Cancer Res. 2019;25(8):2575–87. [DOI] [PubMed] [Google Scholar]
- 12.Ministry of Food and Drug Safety Republic of Korea: Leclaza tablets 80 mg (as lazertinib mesylate monohydrate): Prescribing information [Korean]. 2021.
- 13.Ahn MJ, Han JY, Lee KH, Kim SW, Kim DW, Lee YG, Cho EK, Kim JH, Lee GW, Lee JS, et al. Lazertinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: results from the dose escalation and dose expansion parts of a first-in-human, open-label, multicentre, phase 1–2 study. Lancet Oncol. 2019;20(12):1681–90. [DOI] [PubMed] [Google Scholar]
- 14.Cho BC, Han JY, Kim SW, Lee KH, Cho EK, Lee YG, Kim DW, Kim JH, Lee GW, Lee JS, et al. A phase 1/2 study of lazertinib 240 mg in patients with advanced EGFR T790M-positive NSCLC after previous EGFR tyrosine kinase inhibitors. J Thorac Oncol. 2022;17(4):558–67. [DOI] [PubMed] [Google Scholar]
- 15.Cho BC, Ahn MJ, Kang JH, Soo RA, Reungwetwattana T, Yang JC, Cicin I, Kim DW, Wu YL, Lu S, et al. Lazertinib versus gefitinib as first-line treatment in patients with EGFR-mutated advanced non-small-cell lung cancer: Results from LASER301. J Clin Oncol. 2023;41(26):4208–17. [DOI] [PubMed] [Google Scholar]
- 16.Ahn MJ, Tsai CM, Shepherd FA, Bazhenova L, Sequist LV, Hida T, Yang JCH, Ramalingam SS, Mitsudomi T, Jänne PA, et al. Osimertinib in patients with T790M mutation-positive, advanced non-small cell lung cancer: Long-term follow-up from a pooled analysis of 2 phase 2 studies. Cancer. 2019;125(6):892–901. [DOI] [PubMed] [Google Scholar]
- 17.Papadimitrakopoulou VA, Mok TS, Han JY, Ahn MJ, Delmonte A, Ramalingam SS, Kim SW, Shepherd FA, Laskin J, He Y, et al. Osimertinib versus platinum-pemetrexed for patients with EGFR T790M advanced NSCLC and progression on a prior EGFR-tyrosine kinase inhibitor: AURA3 overall survival analysis. Ann Oncol. 2020;31(11):1536–44. [DOI] [PubMed] [Google Scholar]
- 18.Thress KS, Brant R, Carr TH, Dearden S, Jenkins S, Brown H, Hammett T, Cantarini M, Barrett JC. EGFR mutation detection in ctDNA from NSCLC patient plasma: A cross-platform comparison of leading technologies to support the clinical development of AZD9291. Lung Cancer. 2015;90(3):509–15. [DOI] [PubMed] [Google Scholar]
- 19.Ebert EBF, McCulloch T, Hansen KH, Linnet H, Sorensen B, Meldgaard P. Clearing of circulating tumour DNA predicts clinical response to first line tyrosine kinase inhibitors in advanced epidermal growth factor receptor mutated non-small cell lung cancer. Lung Cancer. 2020;141:37–43. [DOI] [PubMed] [Google Scholar]
- 20.Song Y, Hu C, Xie Z, Wu L, Zhu Z, Rao C, Liu L, Chen Y, Liang N, Chen J, et al. Circulating tumor DNA clearance predicts prognosis across treatment regimen in a large real-world longitudinally monitored advanced non-small cell lung cancer cohort. Transl Lung Cancer Res. 2020;9(2):269–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guan S, Deng G, Sun J, Han Q, Lv Y, Xue T, Ding L, Yang T, Qian N, Dai G. Evaluation of circulating tumor DNA as a prognostic biomarker for metastatic pancreatic adenocarcinoma. Front Oncol. 2022;12: 926260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hallermayr A, Steinke-Lange V, Vogelsang H, Rentsch M, de Wit M, Haberl C, Holinski-Feder E, Pickl JMA. Clinical validity of circulating tumor DNA as prognostic and predictive marker for personalized colorectal cancer patient management. Cancers (Basel). 2022;14(3):851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reichert ZR, Morgan TM, Li G, Castellanos E, Snow T, Dall’Olio FG, Madison RW, Fine AD, Oxnard GR, Graf RP, et al. Prognostic value of plasma circulating tumor DNA fraction across four common cancer types: a real-world outcomes study. Ann Oncol. 2023;34(1):111–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gray JE, Ahn M-J, Oxnard GR, Shepherd FA, Imamura F, Cheng Y, Okamoto I, Cho BC, Lin M-C, Wu Y-L, et al. Early clearance of plasma epidermal growth factor receptor mutations as a predictor of outcome on osimertinib in advanced non–small cell lung cancer; exploratory analysis from AURA3 and FLAURA. Clin Cancer Res. 2023;29(17):3340–51. [DOI] [PubMed] [Google Scholar]
- 25.Wu Y-L, Ahn M-J, Garassino MC, Han J-Y, Katakami N, Kim HR, Hodge R, Kaur P, Brown AP, Ghiorghiu D, et al. CNS efficacy of osimertinib in patients with T790M-positive advanced non–small-cell lung cancer: Data from a randomized phase III Trial (AURA3). J Clin Oncol. 2018;36(26):2702–9. [DOI] [PubMed] [Google Scholar]
- 26.Jang SB, Kim KB, Sim S, Cho BC, Ahn MJ, Han JY, Kim SW, Lee KH, Cho EK, Haddish-Berhane N, et al. Cardiac safety assessment of lazertinib: Findings from patients with EGFR mutation-positive advanced NSCLC and preclinical studies. JTO Clin Res Rep. 2021;2(10): 100224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reungwetwattana T, Cho BC, Lee KH, Pang YK, Fong CH, Kang JH, Lee YG, Lim CS, Danchaivijitr P, Lim YN, et al. Lazertinib versus gefitinib tyrosine kinase inhibitors in treatment-naíve patients with EGFR-mutated advanced NSCLC: Analysis of the Asian subpopulation in LASER301. J Thorac Oncol. 2023;18(10):1351–61. [DOI] [PubMed] [Google Scholar]
- 28.Cho BC, Lu S, Felip E, Spira AI, Girard N, Lee JS, Lee SH, Ostapenko Y, Danchaivijitr P, Liu B, Alip A, Korbenfeld E, Mourão Dias J, Besse B, Lee KH, Xiong H, How SH, Cheng Y, Chang GC, Yoshioka H, Yang JC, Thomas M, Nguyen D, Ou SI, Mukhedkar S, Prabhash K, D'Arcangelo M, Alatorre-Alexander J, Vázquez Limón JC, Alves S, Stroyakovskiy D, Peregudova M, Şendur MAN, Yazici O, Califano R, Gutiérrez Calderón V, de Marinis F, Passaro A, Kim SW, Gadgeel SM, Xie J, Sun T, Martinez M, Ennis M, Fennema E, Daksh M, Millington D, Leconte I, Iwasawa R, Lorenzini P, Baig M, Shah S, Bauml JM, Shreeve SM, Sethi S, Knoblauch RE, Hayashi H, MARIPOSA Investigators. Amivantamab plus lazertinib in previously untreated EGFR-mutated advanced NSCLC. N Engl J Med. 2024. 10.1056/NEJMoa2403614.
- 29.Peled M, Agassi R, Czeiger D, Ariad S, Riff R, Rosenthal M, Lazarev I, Novack V, Yarza S, Mizrakli Y, et al. Cell-free DNA concentration in patients with clinical or mammographic suspicion of breast cancer. Sci Rep. 2020;10(1):14601. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Duration of lazertinib treatment in ctDNA Cleared group and Not Cleared group. Table S2. Drug-related treatment-emergent adverse events occurring in ≥ 10% of patients. Table S3. Ethics committee approvals.
Additional file 2: Fig. S1. Subgroup analyses of overall survival by I mutation type, brain metastases, and brain radiotherapy. Fig. S2. Cumulative incidence rate of CNS progression in lazertinib-treated patients in the LASER201 trial. Fig. S3. Time to first or second subsequent therapy or death in patients with EGFR T790M-positive non-small cell lung cancer. Data cut-off April 8, 2022. Fig. S4. Patient disposition for ctDNA analysis. Patients were categorized based on their clearance status as determined by ddPCR. Fig. S5. Analysis of plasma EGFR mutations at baseline, cycle 3, and cycle 5 in patients with a valid ctDNA sample at baseline and cycle 3 and/or cycle 5. Kaplan–Meier estimates showing OS and independent central review-assessed PFS according to the presence of ctDNA at baseline, cycle 3, and cycle 5. Fig. S6. Resistance mechanism with ctDNA clearance. Fig. S7. Summary of baseline genetic alterations at screening by ctDNA clearance at cycle 3 or not.
Data Availability Statement
Deidentified participant data will be made available when all endpoints have been evaluated. Any requests for trial data and supporting material (data dictionary and statistical analysis plan) will be reviewed by the trial management group in the first instance. Only requests that have a methodologically sound proposal and whose proposed use of the data has been approved by the independent trial steering committee will be considered. Proposals should be directed to the corresponding author in the first instance; to gain access, data requestors will need to sign a data access agreement.