Abstract
MicroRNAs (miRNAs) only recently have been recognized as promising molecules for both fundamental and clinical neuroscience. We provide a literature review of miRNA biomarker studies in three most prominent psychiatric disorders (depression, bipolar disorder and schizophrenia) with the particular focus on depression due to its social and healthcare importance. Our search resulted in 191 unique miRNAs across 35 human studies measuring miRNA levels in blood, serum or plasma. 30 miRNAs replicated in more than one study. Most miRNAs targeted neuroplasticity and neurodevelopment pathways. Various limitations do not allow us to make firm conclusions on clinical potential of studied miRNAs. Based on our results we discuss the rationale for future research investigations of exosomal mechanisms to overcome methodological caveats both in studying etiology and pathogenesis, and providing an objective back-up for clinical decisions.
Electronic supplementary material
The online version of this article (10.1007/s10571-019-00684-6) contains supplementary material, which is available to authorized users.
Keywords: Exosomes, miRNA, Biomarker, Depression, Schizophrenia, Bipolar disorder, Psychiatry
Introduction
Mental disorders are a major concern for healthcare systems worldwide and a significant burden to both an individual and society (Thyloth et al. 2016). Major depressive disorder (MDD) is rated as one of the greatest contributors to global disability, affecting 300 million people worldwide, the main cause of suicide and is not restricted to any particular age group (Mojtabai et al. 2016; Organization 2017). One of the challenges in the management of depression is the lack of definitive diagnostic tools, with multiple studies suggesting a high rate of misdiagnosis in general practice (Mitchell et al. 2009; Pelletier et al. 2017), which may be related to changes in mood disorders section in DSM-5 (Mojtabai 2013; Park and Kim 2018; Uher et al. 2014). In spite of having a wide range of drugs (Girardi et al. 2009; Pompili et al. 2013) for depression the response rate in patients is rather moderate (Undurraga and Baldessarini 2012). There are high hopes for wide use of the non-pharmacologic treatment (e.g. Transcranial Magnetic Stimulation) (Berlim et al. 2013; Serafini et al. 2015) when pharmacologic treatment fails. A substantial minority experiences more disabling conditions such as schizophrenia or bipolar disorder due to their early-onset, chronicity and severity (Ferrari et al. 2016; Moreno-Kustner et al. 2018). Bipolar disorder often shows predominance of depressive symptoms, which makes it difficult for differentiation and therapy (Miller et al. 2014). Due to the above-mentioned epidemiological relevance of depressive disorders and the fact that this article could not solely cover every existing mental illness, only depression will be discussed in detail hereafter. Bipolar disorder and schizophrenia will be discussed in the context of psychiatric illnesses’ biomarkers and in comparison to the biomarker studies in depression.
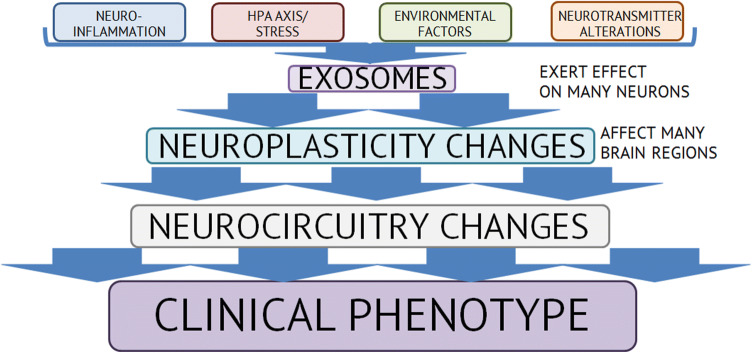
The underlying biological mechanisms of depression and other affective disorders have been described from many perspectives, accounting for the role of stress (Young et al. 2016), inflammation (Brites and FeRNAndes 2015), genetic and environmental factors (Palazidou 2012), alterations in monoamine neurotransmission (Liu et al. 2017), neuroplasticity (Dean and Keshavan 2017). These data gave rise to the speculation that depression presents the dysregulations of these pathways (monoamine and glutamate neurotransmission, neuroinflammation, HPA axis and stress response, brain-derived neurotrophic factor—BDNF) that lead to neuroplasticity changes which disturb neurocircuitry involved in affect regulation which in turn shows up clinically as depressive symptoms (Dean and Keshavan 2017). Abundance in potential pathways has led to identification of dozens of biomarkers with various statistical significance: BDNF (Pinto et al. 2017), IL-6 and IL-1 (Carvalho et al. 2016), cortisol (Boiko et al. 2017), etc. (Carvalho et al. 2016). MiRNAs have shown promising results as potential biomarkers in various fields of medicine (Liu and Lu 2015), however, they are underinvestigated in depression (as well as in other psychiatric disorders), although different studies showed their importance in the central nervous system (Aksoy-Aksel et al. 2014; Wang et al. 2012) and that their levels are frequently altered in mental disorders and change during treatment (Kolshus et al. 2014; Liu et al. 2018).
MiRNAs are one of the most abundant subclasses of small non-coding RNAs with an average length of 19-24 nucleotides (Bartel 2004). Their main function is regulating post-transcriptional gene expression through (1) mRNA degradation, (2) inhibition of translation (Bartel 2004). MiRNAs in eukaryotes downregulate gene expression to keep the activity of various biological pathways at optimal level (Vasudevan 2012). Different studies demonstrated that each miRNA can regulate expression of more than one mRNA and even without full complementarity (Mohr and Mott 2015; Thomson et al. 2011). Neuronal miRNAs account for 70% of all miRNAs in our body and are involved in regulating neurogenesis and neuroplasticity (Nowak and Michlewski 2013). Many researchers suggest that miRNAs are transported throughout the body predominantly in the exosomes (Cheng et al. 2014; Sohel 2016; Tian et al. 2017). The exact miRNA profile of exosomes depends on exosomes’ origin, suggesting tissue-specific miRNA biogenesis and selective sorting of miRNA (Abels and Breakefield 2016; Ha and Kim 2014; Winter et al. 2009). MiRNAs are considered as key functional element of exosomes which could influence cell in two ways: (1) negative regulation of gene expression (Zhang et al. 2015); (2) interaction with cell receptors as ligands (Fabbri et al. 2012). This with the fact of involvement in regulation of nearly every pathway in cell make miRNAs very promising biomarker for disease progression and treatment response.
Exosomes are derived from inward budding of endosomal membrane of the multi-vesicular bodies/endosomes (MVBs/MVEs) vesicles carrying lipids, proteins and nucleic acids that are secreted into the extracellular environment and could be taken up by other cells (van Niel et al. 2018). Exosomes are bilayer membrane extracellular vesicles (EVs) 30-150 nm in size with varying composition (Colombo et al. 2014). They are released by most types of cells and can be found in almost any biological fluid (blood, urine, cerebrospinal fluid, etc.) (Yanez-Mo et al. 2015). The exact composition of exosomes (tetraspanins, cytoskeletal proteins, transport and binding proteins, etc.) depends on many factors such as origin of cell, cellular release site, physiological or pathological state of the cell (Fiandaca et al. 2015; Yanez-Mo et al. 2015), and as a result defines the exact mechanism of exosome-cell interaction (van Niel et al. 2018). The most up-to-date information regarding proteins, lipids and RNAs in exosomes is available on http://www.exocarta.org/, http://www.microvesicles.org/ and http://student4.postech.ac.kr/evpedia2_xe/xe/. Exosomes also play an important role in CNS physiology and pathology as they are a route of local and distant communication between neurons and glia, as well as cells of other organs and tissues (Janas et al. 2016; Von Bartheld and Altick 2011). Banigan et al. showed that schizophrenia and bipolar disorder have distinct exosomal miRNA content derived from post-mortem brain samples (Banigan et al. 2013; Choi et al. 2017). Proteins related to neuron-derived exosomes also showed potential diagnostic utility as blood biomarkers of MDD (Kuwano et al. 2018). All of this combined shows that exosomes and their cargo (especially miRNAs due to their pleiotropic regulation) are intriguing biomarker candidates potentially containing information of clinical importance as well as information elucidating transcriptional networks underlying mental disorders, which can be missed.
The most recent work regarding the role of exosomes in mental disorders by (Saeedi et al. 2019) gives a broad review of potential role of exosomes in mental disorders supporting our idea that exosomes have a huge potential as biomarkers due to their role in neurobiology and their ability to reflect physiological and pathological changes taking place in the brain. We, in turn, provide an overview of miRNAs in exosomes as potentially the most important cargo that is responsible for changes in normal CNS physiology. Initially, we intended to conduct meta-analysis of peripherally measured (serum, plasma, whole blood) miRNA levels in depression and compare them to the bipolar disorder and schizophrenia. However, our literature search revealed substantial discordance in the expression levels of candidate microRNAs among various studies that made conduction of meta-analysis impossible. Therefore, rather than conducting a meta-analysis of potential candidates, this report provides a qualitative summary of the current state of knowledge on specific changes in miRNA profiles in depression, bipolar disorder and schizophrenia, assesses the potential for their use as biomarkers in clinical practice and provides the analysis of how exosomes can facilitate miRNA testing. We also provide information on potential or validated targets of analyzed miRNAs that were found either in the analyzed studies or in the additional literature.
Materials and Methods
For our report, we searched PubMed and Google Scholar databases for all published studies in English through October 1, 2018 using search terms “depression”, “bipolar disorder”, and “schizophrenia” together with their abbreviations and derivatives to include all articles examining miRNAs in relation to any form of depression schizophrenia or bipolar disorder. This search yielded in identifying 302 articles. Out of 302 articles 11 review articles (Alural et al. 2017; Dwivedi 2014; Fries et al. 2018; Geaghan and Cairns 2015; He et al. 2017; Kichukova et al. 2015; Maffioletti et al. 2014; Narahari et al. 2017; Sethi 2017; Sun and Shi 2015; Yuan et al. 2018) were manually searched for any additional relevant studies. Our search resulted in 35 studies that met our criteria: 19 for depression, 3 for bipolar-affective disorder, 11 for schizophrenia, 1 for bipolar and depression, 1 for depression, anxiety or stress and adjustment disorder. The most frequently used miRNA sources were whole blood and plasma (both 14 studies). Serum was chosen as a miRNA source in six studies. Only one study analyzed both blood and plasma levels of a miRNA. Inclusion criteria were: (1) human studies; (2) quantitative analysis of miRNA derived from blood, serum or plasma. Exclusion criteria were: (1) animal studies; (2) other meta-analyses without direct miRNA measurement; (3) peripheral blood mononuclear cell (PBMC) studies; (4) comorbid disorders. The references cited in the analyzed papers were manually searched for any additional relevant studies. Attempts were made to obtain more miRNA expression data from some authors of the selected studies; no additional information was received.
Results
Overall, 237 miRNAs were measured across 35 studies with 191 unique miRNAs. All miRNAs included were significantly differentially expressed either between patients and healthy control or before and after a particular intervention in the analyzed studies (Table 1). More specified information on the studies is provided in Supplementary materials. 30 miRNAS replicated in more than one study (Table 2). In Table 2 we additionally provided information on potential miRNA targets contributing to the development of psychiatric disorder. Intercomparison between studies showed 61 only downregulated, 113 only upregulated and 17 both up- and downregulated miRNAs (Table 3). One miRNA was found differentially expressed in all three disorders, eight were found both in bipolar and depression, one—in bipolar and schizophrenia, and six—in depression and schizophrenia (Table 4). The most popular miRNA level measurement method was quantitative real time PCR (qRT-PCR). Four studies used NGS methods (Gallego et al. 2018; Ma et al. 2018; Wei et al. 2015; Zhang et al. 2018) but only two (Ma et al. 2018; Wei et al. 2015) of them used qRT-PCR as validation method. Only two studies (Gallego et al. 2018; Zhang et al. 2018) thus far measured exosomal miRNA in peripheral specimens.
Table 1.
Details on analyzed studies (extended version in Supplementary Materials)
| Disorder | Source | Patients | Controls | Study design | Ref. |
|---|---|---|---|---|---|
| MDD (?) | Serum | 84 | 78 | DSM-IV depression criteria (no specifications); no antidepressant therapy for new or previous diagnosis of depression within 2 weeks prior to study; all patients were treated with paroxetine; miRNA levels measured at baseline and after 8 weeks of treatment | Kuang et al. (2018) |
| MDD (?) | Plasma | 40 | 40 | CCMD-3 criteria (no specifications); first-time diagnosed, drug-naïve | Li et al. (2013) |
| MDD (?) | Serum | 39 | 36 | DSM-IV depression criteria (no specifications) | Gheysarzadeh et al. (2018) |
| MDD (?)/anxiety or stress/adjustment disorder | Plasma | 169 | 52 | ICD-10 criteria (no specifications); the study compared miRNA levels in a mindfulness-based group therapy program with treatment as usual (mostly individual CBT). Patients received antidepressants (mostly SSRIs) and tranquilizers if necessary | Wang et al. (2015) |
| MDD (?) | Serum (exosomes) | 1 | 1 | No specifications on diagnostic criteria | Zhang et al. (2018) |
| MDD | Plasma | 50 | 41 | DSM-IV criteria; homogenous study group (no comorbid psychiatric or medical conditions; psychotic, melancholic, anxious, seasonal, and atypical subtypes and alcohol users were exclusion criteria) | Camkurt et al. (2015) |
| MDD | Blood | 10 | – | ICD-10 or DSM-IV criteria; 8 drug-naive and 2 went off herbal treatment for at least 2 weeks prior the study; measurements at baseline (T0) and after 12 weeks of escitalopram treatment (T12) | Bocchio-Chiavetto et al. (2013) |
| MDD | Blood | 11 | 11 | No specifications (DSM-IV (?)) | Issler et al. (2014) |
| MDD | Blood | 32 | 18 | DSM-IV criteria; all patients were drug-naïve and were treated with citalopram. MiRNA levels were measured at baseline and 8 weeks of treatment | Lopez et al. (2014) |
| MDD | Blood | 18 | 18 | ICD-10 or DSM-IV criteria | Li et al. (2015) |
| MDD | Plasma | 5 | DSM-IV-TR criteria of MDD and MDE; the study measured miRNA levels at baseline and 12 weeks after the escitalopram treatment | Enatescu et al. (2016) | |
| MDD | Blood | 62 | 73 | DSM-IV criteria; drug-naïve patients | Liu et al. (2016) |
| MDD | Serum | 18 | 17 | DSM-IV criteria; patients were psychotropic drug-free for at least 1 month before blood sampling | Roy et al. (2017a, b) |
| MDD | Blood/plasma |
(1) 258; (2) 61; (3) 158 |
– |
ICD-10/DSM-IV criteria; (1) Discovery cohort (MDD in a current MDE), Duloxetine and placebo groups, baseline and 8 weeks after treatment levels; )2) 1st validation cohort (MDE), Escitalopram and Nortriptyline, baseline and 8 weeks after treatment levels; 3) 2nd validation cohort (MDD), Escitalopram for 8 weeks, baseline and 8 weeks after treatment levels; miR-503-5p was significantly changed in responders and nonresponders in both placebo- and duloxetine-treated patients; all validated miRNAs could be biomarkers of antidepressant response |
Lopez et al. (2017) |
| MDD | Blood |
(1) 55; (2) 124. |
(1) 174; (2) – |
No specifications on diagnostic criteria; miRNA levels were measured at baseline and 8 weeks after treatment in both cohorts; 1st cohort: 27 were treated with escitalopram (SSRI) and 28 with desvenlafaxine (SNRI). Later they were subdivided in 31 responders (15—escitalopram, 16—desvenlafaxine) and 24 nonresponders (12—escitalopram, 12—desvenlafaxine); 2nd cohort: all treated with duloxetine (SNRI), 97 responders and 27 nonresponders |
Fiori et al. (2017) |
| MDD | Plasma | 77 | 32 | DSM-IV criteria | Fang et al. (2018) |
| MDD | Blood | 30 | 30 | Details of inclusion and exclusion criteria were not specified | Su et al. (2015) |
| MDD | Serum |
(1) 6; (2) 32 |
(1) 6; (2) 21 |
ICD-10 or DSM-IV criteria; all patients were antidepressant-free; 2 cohort study (discovery and validation): 1st cohort 6 vs. 6 (CSF and serum); 2nd cohort 32 vs. 21 (serum only) |
Wan et al. (2015) |
| MDD | Blood | 40 | 20 | Treatment- resistant depression; 24 patients received ECT and 16 patients received KET; details of MDD criteria were not specified | Gururajan et al. (2016) |
| MDD | Plasma |
(1) 123; (2) 259 |
(1) 55; (2) 238 |
DSM-IV criteria; 2nd cohort was the expansion of the first one; all the pathologies had dysregulation of NET | Marques et al. (2017) |
| BD type 1 and 2, MDD | Blood | 40 (20 MDD and 20 BD) | 20 | BD and MD patients had current major depressive episode (DSM-IV-TR criteria) | Maffioletti et al. (2016) |
| BD type 1 | Blood | 34 | 46 | Relatives of patients with BD type 1 (first or second-degree relatives; no personal history of BD; high-risk); affected relatives were diagnosed with SCID | Walker et al. (2015) |
| BD type 1 | Blood | 10 | – | DSM-5, manic phase, drug-free, treated with two different antipsychotics (both groups received valproate as a mood stabilizer); baseline levels and after 1, 4, and 12 weeks | Lim et al. (2016) |
| BD | Plasma | 21 | 21 | BD type I, manic phase (DSM-IV), drug-free, antipsychotic and mood stabilizer combination | Rong et al. (2011) |
| SCZ | Blood | 16 | 16 | DSM-IV-TR criteria; all patients were in active psychotic episode. Three patients were drug-naïve and had first episode, and 13 patients were previously on atypical antipsychotic treatment | Camkurt et al. (2016) |
| SCZ | Blood | 44 | 44 | DSM-IV criteria; all patients were first-onset | Ma et al. (2018) |
| SCZ | Plasma | 40 | – | The patients were all treated with risperidone and achieved remission in 1 year. No specifications on diagnosis of schizophrenia. | Liu et al. (2013) |
| SCZ | Plasma | 61 | 62 | DSM-IV schizophrenia criteria; Patients were either drug-naïve or absent of psychotropic medication within at least 3 months. 25 patients received a 6-week atypical antipsychotic treatment (miRNA levels were evaluated before medication, and 3 and 6 weeks after); olanzapine had the strongest effect on plasma miRNA level changes; 10 miRNAs were selected according to the latest information in PubMed database, Springer database and the University of British Columbia library database | Sun et al. (2015a, b) |
| SCZ | Plasma | 25 | 13 | DSM-IV criteria, all patients were selected prior to any antipsychotic treatment, or in the absence of treatment for at least 3 months prior to the study; 9 miRNAs were selected according to the latest information in PubMed database, Springer database and the University of British Columbia library database | Sun et al. (2015a, b) |
| SCZ | Plasma | 37 | 10 | DSM-IV criteria | Alacam et al. (2016) |
| SCZ | Serum | 115 | 40 | Paranoid and undifferentiated schizophrenia (ICD-10). Patients were classified into schizophrenia subtypes, with family history or not. All the patients were treated. 9 miRNAs were chosen according to the literature, Schizophrenia Gene database, and NCBI database | Shi et al. (2012) |
| SCZ | Plasma | 20 | 20 | DSM-IV criteria; Before and after treatment evaluation (4 groups of 5 patients in each); all patients were treated with atypical antipsychotics; 9 miRNAs were selected according to the latest information in PubMed database, Springer database and the University of British Columbia library database | (Song et al. (2014) |
| SCZ | Plasma | 726 | – |
DSM-IV criteria; all participants were divided into two cohorts: (1) test cohort (164 with schizophrenia and 187 controls); (2) validation cohort (400 with schizophrenia, 213 controls, and 162 with nonschizophrenia disorders). 107 patients from validation cohort reached study endpoint, which was 12 months of treatment with atypical antipsychotics (risperidone and aripiprazole) |
Wei et al. (2015) |
| SCZ | Blood | 44 | 44 | DSM-IV criteria; all patients were drug-naïve. | Wu et al. (2016) |
| SSD | Plasma (EVs) | 22 | 17 | Patients: 16 (72.7%) had schizophrenia, 5 (22.7%) had schizoaffective disorder and 1 (4.5%) had psychosis not otherwise specified: details or specifications regarding diagnostic criteria were not specified | Gallego et al. (2018) |
? means that there are no clear specifications on whether MDD diagnosis was established
SCZ schizophrenia, SSD schizophrenia spectrum disorder, BD bipolar disorder, MDE major depressive episode, MDD major depressive disorder, SCID structured clinical interview for DSM-IV, DSM-IV diagnostic and statistical manual of mental disorders, Fourth edition, DSM-IV-TR diagnostic and statistical manual of mental disorders, Fourth edition, Text revision, CCMD-3 Chinese classification of mental disorders, DSM-5 diagnostic and statistical manual of mental disorders, Fifth edition, ICD-10 inteRNAtional statistical classification of diseases and related health problems 10th revision, CSF cerebrospinal fluid, NET norepinephrine transporter, ECT electroconvulsive therapy, KET ketamine, CBT cognitive behavioral therapy, SSRI selective serotonin reuptake inhibitors, SNRI serotonin–norepinephrine reuptake inhibitors, EVs extracellular vesicles
Table 2.
Frequently assessed miRNAs and their potential targets and mechanisms in psychiatric disorder pathogenesis (extended version is available in Supplementary Materials)
| MiRNA | Number of studies | Disorder | Source | Expression | Potential target/mechanism | Target ref. |
|---|---|---|---|---|---|---|
| miR-132 | 8 | BD type 1 | Blood | ↑ | BDNF; inflammation, immune functions | Wanet et al. (2012), Yuan et al. (2018) |
| SCZ | Plasma | ↓ | ||||
| SCZ | Plasma | ↑ | ||||
| MDD | Plasma | ↑ | ||||
| MDD | Blood | ↑ | ||||
| MDD | Plasma | ↑ | ||||
| MDD | Blood | ↑ | ||||
| MDD | Blood | ↑ | ||||
| miR-181b | 4 | SCZ | Serum | ↑ | VSNL1, GRIA2; Signaling pathways: Wnt/β-catenin, cancer, endocytosis, MAPK | Beveridge et al. (2008), Enatescu et al. (2016) |
| SCZ | Plasma | ↑ | ||||
| SCZ | Plasma | ↑ | ||||
| MDD | Plasma | ↓ | ||||
| miR-30e | 3 | SCZ | Plasma | ↑ | Neuronal survival, neuroinflammation; Signaling pathways: Wnt/β-catenin, Axon guidance, IGF1, NGF | Labouesse et al. (2018), Li et al. (2018a, b), Lu et al. (2017), Wang et al. (2013) |
| SCZ | Plasma | ↑ | ||||
| SCZ | Plasma | ↑ | ||||
| miR-7 | 3 | SCZ | Plasma | ↑ | SHANK3, targets interacting with SHANK3 in actin-regulatory pathway. Neuroprotection; inflammation; PTEN pathway; neurite outgrowth, synapse formation, brain development | Chakrabarti et al. (2016), Choi et al. (2015), Garbett et al. (2015), Prata et al. (2017) |
| SCZ | Plasma | ↑ | ||||
| SCZ | Plasma | ↑ | ||||
| miR-451a | 3 | MDD | Plasma | ↑ | SLC17A7 | Our target prediction. |
| MDD | Serum | ↓ | ||||
| MDD | Serum | ↓ | ||||
| miR-195 | 3 | SCZ | Serum | ↓ | BDNF, RELN, VSNL1, HTR2A, HTR4, GPRIN3 | Beveridge et al. (2010), Butler et al. (2016), Guo et al. (2010) |
| SCZ | Plasma | ↓ | ||||
| SCZ | Plasma | ↑ | ||||
| miR-146b-5p | 3 | BD type 1 | Blood | ↓ | Signaling pathways (MAPK and Wnt), BDNF | Enatescu et al. (2016), Hsu et al. (2015), Lopez et al. (2017) |
| MDD | Plasma | ↓ | ||||
| MDD | Blood/plasma |
(1) ↓ in both placebo and duloxetine-treated patients; (2) ↓ only in response group; (3) ↓ only in response group. |
||||
| miR-1202 | 3 | MDD | Blood | ↓ | GRM4 | Fiori et al. (2017) |
| MDD | Blood | ↓ | ||||
| MDD | Serum | ↓ | ||||
| miR-135a | 3 | MDD | Blood | ↓ | SERT, HTR1A, Cplx1, Cplx2 | Issler et al. (2014), Mannironi et al. (2018) |
| MDD | Blood | |||||
| MDD | Serum | ↓ | ||||
| miR-652 | 2 | BD type 1 | Blood | ↑ | Signaling pathways (Wnt, axon guidance, and endocytosis); pathways and targets associated with BD, schizophrenia and schizoaffective disorder (GABA receptor subunits, 5-HT1D, DISC1 and Reelin signaling); immune system and oxidative stress pathways | Enatescu et al. (2016), Hsu et al. (2015), Liguori et al. (2018), Walker et al. (2015) |
| MDD | Plasma | ↓ | ||||
| miR-425-3p | 2 | MDD | Blood | ↑ | MAPK/Wnt signaling pathways | Lopez et al. (2017) |
| MDD | Blood/plasma |
(1) ↓ in both placebo and duloxetine-treated patients; (2) ↓ only in response group; |
||||
| miR-375 | 2 | MDD | Plasma | ↑ | BDNF | Wan et al. (2015) |
| SSD | Plasma (EVs) | ↑ | ||||
| miR-34a-5p | 2 | MDD | Serum | ↑ | BDNF. SHANK3 and potentially other targets interacting with SHANK3 in actin-regulatory pathway | Bavamian et al. (2015), Choi et al. (2015), Dwivedi, (2011), Wan et al. (2015) |
| MDD | Serum | ↑ | ||||
| miR-34a | 2 | SCZ | Plasma | ↑ | BDNF. SHANK3 and potentially other targets interacting with Shank3 in actin-regulatory pathway. | Bavamian et al. (2015), Choi et al. (2015), Dwivedi, (2011), Wan et al. (2015) |
| SCZ | Plasma | ↑ | ||||
| miR-346 | 2 | SCZ | Serum | ↑ | GRID1, circadian rhythm | Bavamian et al. (2015), Figueredo et al. (2013) |
| SCZ | Plasma | ↑ | ||||
| miR-335 | 2 | MDD | Blood | ↑ | GRM4, synaptic plasticity | Capitano et al. (2017), Li et al. (2015) |
| MDD | Blood | ↓ | ||||
| miR-26a | 2 | MDD | Blood | ↑ | BDNF, signaling pathways (Wnt signaling, Cancer, Endocytosis, Axon guidance and MAPK signaling) | Caputo et al. (2011), Enatescu et al. (2016) |
| MDD | Plasma | ↓ | ||||
| miR-24-3p | 2 | MDD | Blood | ↑ | SERT. Wnt signaling, Cancer, Endocytosis, Axon guidance, MAPK signaling. | Enatescu et al. (2016), Liao et al. (2016), Lopez et al. (2017) |
| MDD | Blood/plasma |
(1) ↓ in placebo and duloxetine-treated patients; (2) ↓ only in response group; (3) ↓ only in response group. |
||||
| miR-221-3p | 2 | MDD | Serum | ↑ | Gja1 (Cnx43) | Shen et al. (2018) |
| MDD | Serum | ↑ | ||||
| miR-182 | 2 | MDD | Plasma | ↑ | BDNF, circadian rhythms | Li et al. (2016a, b), Li et al. (2013), Saus et al. (2010) |
| MDD | Blood | ↑ | ||||
| miR-17-5p | 2 | BD type 1 | Blood | ↑ | CREB1, CHRM2, NTRK3, NPAS3, immune system, circadian rhythm, hippocampal neurogenesis | Chen et al. (2018), Cox et al. (2010), Gao et al. (2016), Jin et al. (2016), Roy et al. (2017a, b), Wong et al. (2013) |
| MDD | Plasma | ↑ | ||||
| miR-144-5p | 2 | MDD/Anxiety or stress/Adjustment disorder | Plasma | ↓ | PKC, Wnt/β-catenin, PTEN pathways, ATXN1 | Dwivedi (2014), Liu and Su (2018) |
| SSD |
Plasma (EVs) |
↑ | ||||
| miR-140-3p | 2 | BD type 1 & 2 | Blood | ↑ | Synaptic plasticity, immune system | Cirnigliaro et al. (2017) |
| MDD | Blood | ↑ | ||||
| miR-130b | 2 | SCZ | Plasma | ↑ | Schizophrenia susceptibility genes (PDGFRA, PPARG), neurodevelopment-related genes (RUNX3, ITGB1, FMR1, STAT3). PTEN, Wnt/β-catenin pathways | Gu et al. (2018), Li et al. (2016a, b), Wei et al. (2015) |
| MDD | Blood | ↑ | ||||
| miR-106b-5p | 2 | BD type 1 | Blood | ↑ | Neurogenesis, PTEN, Wnt/β-catenin | Brett et al. (2011), Li et al. (2017), Lu et al. (2017) |
| SCZ | Blood | ↑ | ||||
| let-7g | 2 | SCZ | Serum | ↑ | Glutamate signaling (SLC38A2, GRM7) | Roy et al. (2017a, b) |
| MDD | Blood | ↑ | ||||
| let-7d | 2 | MDD | Blood | ↑ | Dopamine metabolism, synapse maturation, growth, and dendritic arborization, neurogenesis. Wnt, cancer, MAPK signaling, endocytosis, actin cytoskeleton pathways | Chandrasekar and Dreyer (2011), Enatescu et al. (2016), Mendes-Silva et al. (2016), Wu et al. (2015), Zhao et al. (2013) |
| MDD | Plasma | ↓ | ||||
| miR-210-3p | 2 | MDD | Serum (exosomes) | ↓ | Target miRNA gene (BNIP3) involved in cell death/survival, autophagy, antistress reaction factor, depression, potential role in maintenance and/or reformation of synapses; angiogenesis and neurogenesis in brain tissue repair and remodeling after brain injury | Ivan et al. (2014), Tohda et al. (2010), Zeng et al. (2014) |
| BD type 1 | Whole blood | ↑ | ||||
| miR-16 | 2 | MDD | Serum | ↓ | Regulates SERT via raphe nuclei and hippocampal responses to antidepressants; regulation of apoptosis and autophagy | Bai et al. (2012), Shao et al. (2018), Yang et al. (2017) |
| MDD | Whole blood | |||||
| miR-195-5p | 2 | MDD | Serum (exosomes) | ↑ | HTR2A, BDNF; DRD1a, GRIA3a, VEGFAa, FASNa, APPa, GSK3Ba | Alacam et al. (2016), Beveridge et al. (2010), Zhang et al. (2018) |
| Schizophrenia | Plasma |
SCZ schizophrenia, SSD schizophrenia spectrum disorder, BD bipolar disorder, MDD major depressive disorder, EVs extracellular vesicles, BDNF brain-derived neurotrophic factor, MAOA monoamine oxidase A, SLC6A3 solute carrier family 6 member 3, CLOCK clock circadian regulator, GRM3 glutamate metabotropic receptor 3, SYN2 synapsin II, VSNL1 visinin like 1 protein, GRIA2 glutamate ionotropic receptor AMPA type subunit 2, MAPK mitogen-activated protein kinase, SHANK3 SH3 and multiple ankyrin repeat domains 3, SLC17A7 solute carrier family 17 member 7, RELN reelin, HTR2A 5-hydroxytryptamine receptor 2A, HTR4 5-hydroxytryptamine receptor 4, GPRIN3 GPRIN family member 3, GRM4 glutamate metabotropic receptor 4, SERT serotonin transporter, 5-HT1a 5-hydroxytryptamine receptor 1A, 5-HT1D 5-hydroxytryptamine receptor 1D, GRID1 glutamate ionotropic receptor delta type subunit 1, CREB1 cAMP responsive element binding protein 1, CHRM2 cholinergic receptor muscarinic 2, NTRK3 neurotrophic receptor tyrosine kinase 3, NPAS3 neuronal PAS domain protein 3, PKC protein kinase C, PTEN phosphatase and tensin homolog, ATXN1 ataxin 1, PDGFRA platelet derived growth factor receptor alpha, PPARG peroxisome proliferator activated receptor gamma, RUNX3 runt related transcription factor 3, ITGB1 integrin subunit beta 1, FMR1 fragile X mental retardation 1, STAT3 signal transducer and activator of transcription 3, SLC38A2 solute carrier family 38 member 2, GRM7 glutamate metabotropic receptor 7, IGF1 insulin growth factor 1, NGF nerve growth factor, Cplx1 complexin-1, Cplx2 complexin-2, DISC1 DISC1 scaffold protein, Gja1 (Cnx43) gap junction protein, alpha 1 (connexin 43), BNIP3 BCL2/adenovirus E1B 19 kDa protein-interacting protein 3, DRD1 dopamine receptor D1, GRIA3 glutamate ionotropic receptor AMPA type subunit 3, VEGFA vascular endothelial growth factor A, FASN fatty acid synthase, APP amyloid beta precursor protein, GSK3B glycogen synthase kinase 3 beta
aWe have not found evidences of these miRNA targets in provided references in Zhang et al. (2018)
Table 3.
Different expression levels of miRNAs in analyzed studies
| Expression | Up and down | Down | Up | |||
|---|---|---|---|---|---|---|
| miRNAs | miR-195 | miR-134 | miR-19a-3p | miR-720 | miR-125b-3p | miR-2278 |
| miR-132 | miR-1915- 5p | miR-146a-5p | miR-1973 | miR-137 | miR-3150a-3p | |
| miR-335 | miR-1972 | miR-3074-5p | miR-3158-3p | miR-22-3p | miR-3909 | |
| miR-451a | miR-4793-3p | miR-499a-5p | miR-4521 | miR-92a-3p | miR-937 | |
| miR-144-5p | miR-4440 | miR-4732-3p | miR-140-3p | miR-182 | miR-676* | |
| miR-181b | miR-1915-3p | miR-222-5p | miR-330-5p | miR-505 | miR-489 | |
| miR-26a | let-7a-5p | miR-1291 | miR-378a-5p | miR-29b-2 | miR-637 | |
| let-7d | let-7d-5p | miR-668-3p | miR-30d-5p | miR-26b | miR-608 | |
| miR-652 | let-7f-5p | miR-6511a-3p | miR-21-3p | miR-22 | miR-4263 | |
| miR-24-3p | miR-92b-5p | miR-145-3p | miR-199a-5p | miR-664 | miR-382 | |
| miR-425-3p | miR-1343-5p | miR-200a-3p | miR-29c-5p | miR-494 | miR-3691-5p | |
| miR-210-3p | miR-664b-5p | miR-143-3p | miR-330-3p | let-7e | miR-375 | |
| miR-16 | miR-6778-5p | miR-196b-5p | miR-345-5p | let-7f | miR-433 | |
| miR-135a | miR-146b-5p | miR-99a-5p | miR-15b | miR-629 | miR-1298 | |
| miR-195-5p* | miR-17 | miR-144-3p | miR-18a-5p | miR-106b | miR-1909 | |
| miR-181b-5p* | miR-365 | miR-584-5p | miR-19b-3p | miR-103 | miR-1471 | |
| miR-301a-3p* | miR-520c-3p | miR-183-5p | miR-145-5p | miR-191 | miR-124-3p | |
| miR-432 | miR-107 | miR-27a-3p | miR-128 | miR-204-5p | ||
| miR-770-5p | miR-130b-5p | miR-148b-3p | miR-502-3p | miR-942-5p | ||
| miR-34c-5p | miR-589-5p | miR-17-3p | miR-374b | miR-6734-5p | ||
| miR-1202 | miR-1910-5p | miR-30b-5p | miR-30d | miR-423-5p | ||
| miR-320a | miR-106b-5p | miR-500 | miR-124 | |||
| miR-583 | miR-339-5p | miR-589 | miR-1255a | |||
| miR-650 | miR-106a-5p | miR-183 | miR-3161 | |||
| miR-708 | miR-20a-5p | miR-574-3p | miR-99a-3p | |||
| miR-654 | miR-17-5p | miR-361-5p | miR-205-5p | |||
| miR-151-5p | miR-15a-5p | miR-223-3p | miR-26a-1-3p | |||
| miR-99b | miR-1308 | miR-644 | miR-139-5p | |||
| miR-223 | let-7g | miR-450b | miR-7849-3p | |||
| miR-744 | miR-219-2-3p | miR-328 | miR-125b-2-3p | |||
| miR-301b | miR-346 | miR-221-3p | miR-664a-3p | |||
| miR-27a | miR-92a | miR-34a-5p | let-7c-5p | |||
| miR-24 | miR-30e | let-7d-3p | miR-197-3p | |||
| miR-146a | miR-34a | miR-1193 | ||||
| miR-126 | miR-7 | miR-3173-3p | ||||
| miR-151-3p | miR-130b | miR-3154 | ||||
| miR-221 | miR-193a-3p | miR-129-5p | ||||
| miR-125a-5p | miR-9-5p | miR-3661 | ||||
| let-7b | miR-29a-3p | miR-1287 | ||||
| let-7c | miR-125a-3p | miR-532-3p | ||||
| Total | 17 | 61 | 113 | |||
*Differentially expressed in one study
Table 4.
Overlaps in miRNAs between disorders
| Disorders | BD and MDD and SCZ | BD and MDD | BD and SCZ | MDD and SCZ | BD | MDD | SCZ | ||
|---|---|---|---|---|---|---|---|---|---|
| miRNAs | miR-132 | miR-140-3p | miR-106b-5p | miR-195-5p | miR-134 | miR-1915-3p | miR-650 | miR-151-3p | miR-1308 |
| miR-146b-5p | miR-181b | miR-720 | let-7a-5p | miR-708 | miR-221 | miR-219-2-3p | |||
| miR-17-5p | miR-144-5p | miR-1973 | let-7d-5p | miR-654 | miR-125a-5p | miR-346 | |||
| miR-210-3p | let-7g | miR-3158-3p | let-7f-5p | miR-644 | let-7b | miR-92a | |||
| miR-29c-5p | miR-130b | miR-4521 | miR-24-3p | miR-450b | let-7c | miR-195 | |||
| miR-330-3p | miR-375 | miR-330-5p | miR-425-3p | miR-328 | miR-19a-3p | miR-17 | |||
| miR-345-5p | miR-378a-5p | miR-199a-5p | miR-221-3p | miR-124-3p | miR-30e | ||||
| miR-652 | miR-30d-5p | miR-182 | miR-34a-5p | miR-146a-5p | miR-34a | ||||
| miR-21-3p | miR-770-5p | let-7d-3p | miR-3074-5p | miR-7 | |||||
| miR-1915-5p | miR-34c-5p | miR-1193 | miR-16 | miR-193a-3p | |||||
| miR-1972 | miR-505 | miR-3173-3p | miR-124 | miR-9-5p | |||||
| miR-4793-3p | miR-29b-2 | miR-3154 | miR-1255a | miR-29a-3p | |||||
| miR-4440 | miR-26b | miR-129-5p | miR-3161 | miR-125a-3p | |||||
| miR-15b | miR-22 | miR-3661 | miR-99a-3p | miR-125b-3p | |||||
| miR-18a-5p | miR-26a | miR-1287 | miR-205-5p | miR-137 | |||||
| miR-19b-3p | miR-664 | miR-532-3p | miR-26a-1-3p | miR-22-3p | |||||
| miR-145-5p | miR-494 | miR-2278 | miR-139-5p | miR-92a-3p | |||||
| miR-27a-3p | let-7d | miR-3150a-3p | miR-7849-3p | miR-365 | |||||
| miR-148b-3p | let-7e | miR-3909 | miR-125b-2-3p | miR-520c-3p | |||||
| miR-17-3p | let-7f | miR-937 | miR-664a-3p | miR-432 | |||||
| miR-30b-5p | miR-629 | miR-676* | let-7c-5p | miR-204-5p | |||||
| miR-339-5p | miR-106b | miR-489 | miR-197-3p | miR-942-5p | |||||
| miR-106a-5p | miR-103 | miR-637 | miR-499a-5p | miR-6734-5p | |||||
| miR-20a-5p | miR-191 | miR-608 | miR-4732-3p | miR-423-5p | |||||
| miR-15a-5p | miR-128 | miR-4263 | miR-222-5p | miR-181b-5p | |||||
| miR-92b-5p | miR-502-3p | miR-382 | miR-1291 | miR-301a-3p | |||||
| miR-1343-5p | miR-374b | miR-3691-5p | miR-668-3p | ||||||
| miR-664b-5p | miR-30d | miR-433 | miR-6511a-3p | ||||||
| miR-6778-5p | miR-500 | miR-1298 | miR-145-3p | ||||||
| miR-589 | miR-1909 | miR-200a-3p | |||||||
| miR-183 | miR-1471 | miR-143-3p | |||||||
| miR-574-3p | miR-151-5p | miR-196b-5p | |||||||
| miR-335 | miR-99b | miR-99a-5p | |||||||
| miR-361-5p | miR-223 | miR-144-3p | |||||||
| miR-135a | miR-744 | miR-584-5p | |||||||
| miR-1202 | miR-301b | miR-183-5p | |||||||
| miR-320a | miR-27a | miR-107 | |||||||
| miR-451a | miR-24 | miR-130b-5p | |||||||
| miR-223-3p | miR-146a | miR-589-5p | |||||||
| miR-583 | miR-126 | miR-1910-5p | |||||||
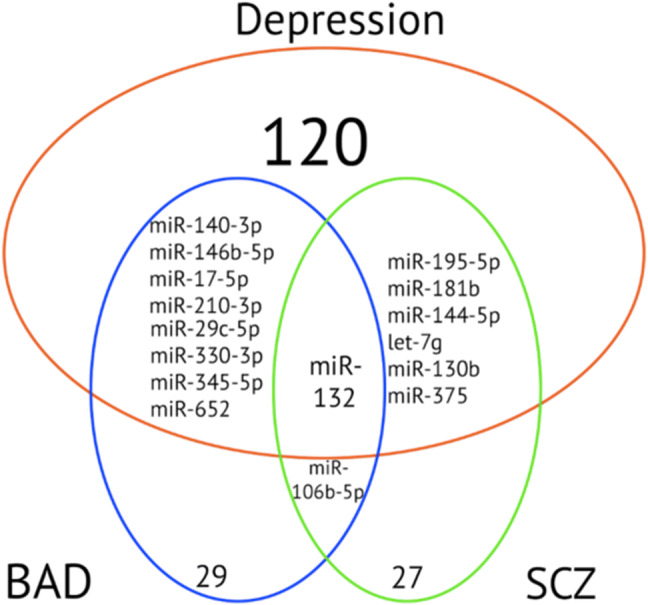
| Total | 1 | 8 | 1 | 6 | 29 | 120 | 27 | ||
BD bipolar disorder, MDD major depressive disorder, SCZ schizophrenia
Diverse study designs, miRNA isolation and quantification protocols have led to not entirely consistent results (see “Methodological Limitations” section below). This fact and inability to look thoroughly through expression data from all studies restricted the possibility of adequate intercomparison, and therefore, we analyzed and compared only readily available data provided in the articles’ texts, i.e., miR-17, miR-17-3p, miR-17-5p were considered three individual cases and therefore analyzed separately. This resulted in identification of 30 miRNAs that were replicated in more than one study (Table 2). More detailed information is available in Supplementary Materials, Tables 1 and 2.
Discussion
Most of the miRNA targets were directly or indirectly involved in different pathologic pathways in depression particularly regarding neuroplasticity and brain development (Table 2). Immune system, inflammation, metabolism of neuromediators, circadian rhythms, other signaling pathways (Wnt, endocytosis, etc.) were also targeted. Same pathway could be targeted by different miRNAs from different disorders as well as the same miRNA could be implicated in several disorders. All three disorders showed overlaps in miRNAs, targeted genes and pathways (Fig.1 and Tables 2 and 4).
Fig. 1.
Overlaps between disorders in miRNAs replicated in more than one study. BD bipolar disorder, MDD major depressive disorder, SCZ schizophrenia
Most of the MDD-related miRNAs targeted pathways or genes related to the neuroplasticity and neurodevelopment (e.g., BDNF). Goldie et al. argued that exosomes with miRNAs are an important component in regulation of neuronal plasticity (Goldie et al. 2014). They proposed that neuronal exosomes facilitate control of synapses. The disruption of this complex mechanism could lead to inadequate control of synaptic plasticity with further suppression or even loss of signal transduction which could be observed in neuropsychiatric pathologies. This suggestion was based on finding of synaptic signal transduction deficit and over-abundance of cortical miRNAs in patients with schizophrenia (Beveridge et al. 2010; Santarelli et al. 2011). MiRNAs in astrocyte-derived exosomes and microglia-derived EVs (exosomes and MVs) were shown to be potential regulators of neuroplasticity (Lafourcade et al. 2016; Prada et al. 2018).
Other popular targets were related to glutamatergic system (GRM4, SLC17A7). Alterations in it contribute to development of neuropsychiatric disorders (Niciu et al. 2012) particularly GRM4 to depression risk (Dadkhah et al. 2017). Serotoninergic system was also targeted by miRNAs (SERT, 5HT1A, 5HT1D). MiRNAs regulating different signaling pathways associated with depression (MAPK and Wnt signaling) were consistently altered. Interestingly, the same miRNAs were altered in BD. To a lesser extent, components of immune system and circadian rhythm were targets of miRNAs although disruption in circadian clock can contribute to the development of psychiatric disorders (Karatsoreos 2014). MDD shared similarities with schizophrenia in same miRNAs targeting BDNF, signaling pathways, glutamatergic system and ATXN1. Although ATXN1 showed to be implicated in all disorders (Liu and Su 2018), miRNAs regulating this gene were altered only in MDD and schizophrenia. There were also miRNAs that were altered only in depression (miR-451a, miR-1202, miR-135a, miR-425-3p, miR-34a-5p, miR-335, miR-26a, miR-24-3p, miR-221-3p, miR-182, let-7d), but one miRNA (miR-451a) showed discordance in expression levels (Camkurt et al. 2015; Kuang et al. 2018; Wan et al. 2015). This could be due to different sources of miRNA (downregulation was observed in serum in contrast to upregulation in plasma) or due to different approach in sampling (pointed out by Camkurt et al. 2015).
Our analysis showed that there are miRNAs exclusively altered in schizophrenia (miR-30e, miR-7, miR-195, miR-34a, and miR-346). They control neuron survival, neuroinflammation, components of glutamatergic system and various psychiatry-related genes. However, schizophrenia showed overlaps with two other disorders.
Therefore, MDD shows similarities predominantly with BD in pathways related to Wnt and MAPK signaling, axon guidance, endocytosis, BDNF, regulation of immune system, synaptic plasticity, circadian rhythm, hippocampal neurogenesis and also in targets associated with BD, schizophrenia and schizoaffective disorder (GABA receptor subunits, 5-HT1D, DISC1 and Reelin signaling). MDD shared pathways and targets with schizophrenia in Wnt/β-catenin, cancer, endocytosis, MAPK, PTEN signaling pathways, calcium-sensor proteins (VSNL1), BDNF, HTR2A, glutamate signaling (SLC38A2, GRM7, GRIA2), neurodevelopment-related genes (RUNX3, ITGB1, FMR1, STAT3) and schizophrenia susceptibility genes (PDGFRA, PPARG). Different miRNAs were altered in different disorders and regulated different targets nevertheless they were involved in the same pathways shared between all three disorders. The overlap between all three disorders was shown in miR-652: although, it was altered only in BD and MDD studies, it regulates pathways and targets associated with BD, schizophrenia and schizoaffective disorder (Walker et al. 2015).
We acquired interesting results regarding miR-34a and miR-34a-5p. These miRNAs showed similar behavior in two different disorders: it was upregulated compared to controls in two schizophrenia studies (Song et al. 2014; Sun et al. 2015a, b) and showed tendency to downregulation after treatment (Song et al. 2014) and the same was shown for depression (Kuang et al. 2018; Wan et al. 2015) [but with significant downregulation after treatment (Kuang et al. 2018)]. Although the results are promising there is a difference between these groups: in depression studies the mature product of miRNA was measured (miR-34a-5p) but not in schizophrenia studies (miR-34).
MiR-132 is the most replicated miRNA (8 studies) but showed varied expression. This data is consistent with the results of Yuan et al. (2018). They explain such reproducibility due to miR-132 regulation of BDNF via methyl CpG-binding protein 2 (MECP2) gene (Su et al. 2015). BDNF is essential for neuronal growth and survival (Tapia-Arancibia et al. 2004). However, we need to mention that proposed explanation for the role of miR-132/BDNF interaction in MDD is very hazy. MiR-132 has been shown to be involved in regulating many genes and processes (Liu et al. 2015; Salta and De Strooper 2017; Wang and Liang 2015). On our side, we analyzed miR-132 targets using online databases for miRNA targets miRDB (http://mirdb.org/) and genes affiliated with all three disorders using the MalaCards human disease database (http://www.malacards.org/). The analysis showed that at least two genes (MAO A and SLC6A3) are targets of miR-132 and associated with all three disorders. This was also true for other genes (CLOCK in depression and BD; GRM3 and SYN2 in schizophrenia and BD).
Therefore, various potential targets of different miRNAs (including our results) should be very carefully interpreted as most of these targets have not been validated via four step protocol involving (1) in silico target prediction; (2) miRNA–target interaction screening; (3) verification of direct interaction (4) in vivo gene expression measurement (Varendi et al. 2015). Thus, there is a chance that some miRNA interactions could be false positive. Most notably is that Varendi et al. showed controversies in miRNA-BDNF (including miR-26a, miR-132, miR-182 and miR-195) relationships which makes us cautious in our conclusions.
There are also problems regarding BDNF itself. Several studies have shown that difference in BDNF peripheral levels exist between depressed compared to healthy and that BDNF levels change after treatment with antidepressants (Jiang et al. 2017,; Molendijk et al. 2014; Polyakova et al. 2015). However, most of BDNF studies have methodologic flaws and BDNF plasma levels alterations were found in other psychiatric disorders as well (FeRNAndes et al. 2015a, b; Molendijk et al. 2012; Polyakova et al. 2015; Suliman et al. 2013) as well as in other diseases (Pichler et al. 2017; Salta and De Strooper 2017; Walker et al. 2015; Weber et al. 2017; Yu et al. 2015). Table 2 contains information regarding other replicated miRNAs and their targets.
The only study provided information regarding exosomal miRNAs was (Zhang et al. 2018).
Methodological Limitations
Recent data warns us that currently available techniques and protocols are unable to precisely differentiate between sub-populations of exosomes, and therefore, we are unable to say what is responsible for any given effect (van Niel et al. 2018; Willms et al. 2016). The purity of exosome pellet directly affects the number of false-positive results in functional analyses (van Niel et al. 2018). There is also a lot to clarify in miRNA-EVs (including exosomes) relationships: (a) whether response in a cell is mediated by induction of endogenous miRNA expression or by the miRNA brought by EVs; (b) how EVs unload their RNA cargo inside the cell; (c) in what form miRNAs are delivered; (d) whether all the EVs contain miRNAs or particular sub-population; etc. (for more information about EVs-RNA relationship look up at (Chevillet et al. 2014; Mateescu et al. 2017, Tkach and Thery 2016 and references therein).
There is a problem regarding exosome identification as previously considered “exosome markers” are present in all types of extracellular vesicles which opens up a discussion about validity of different findings in ‘exosomes’ (Kowal et al. 2016). Therefore, we should consider using guidelines proposed by Kowal et al. as well as the recommendations of InteRNAtional Society for Extracellular Vesicles (ISEV) for precise interpretation of data regarding biology of exosomes (Clayton et al. 2018; Kowal et al. 2016; Mateescu et al. 2017).
Another confusion in miRNA-EV relationship is that miRNAs could be transported by other means (Miller and Raison 2016; Scott 2017; Vickers et al. 2011, Zhang et al. 2015). This in couple with the fact that low-density lipoproteins mimic exosomes (Sodar et al. 2016) is a serious obstacle in isolating pure exosomes and important miRNAs could potentially be missed. Tosar et al. also showed that miRNAs represent a minor fraction of RNAs inside different EVs (including exosomes) (Tosar et al. 2015), and different other types of nucleic acids were identified inside exosomes: mRNA, rRNA, tRNA, DNA, etc., (Lazaro-Ibanez et al. 2014; Li et al. 2014; Tamkovich et al. 2016). These conflicting data shows that our emphasis on miRNAs as principal gene expression regulators is speculative and oversimplification at this time due to a huge number of various non-coding RNAs (ncRNAs) such as PIWI-interacting RNAs (piRNAs), small nucleolar ncRNAs, transcribed ultraconserved region (T-UCRs), and large intergenic ncRNAs which are also involved in regulating cellular pathways (Hombach and Kretz 2016). As we also mentioned before, potential targets of different miRNA (including our results) should be validated which can help understand what particular pathways are altered and how they correlate with clinical symptoms. Nevertheless, ncRNAs fall outside the scope of this study although this controversy should be clarified acknowledged and clarified in future studies.
There are different issues in miRNA studies in psychiatry that does not allow making unambiguous conclusions: (1) small proportion of analyzed miRNAs, significant miRNAs could potentially be missed; (2) possible influence of previous psychotropic drug intake; (3) inability to tell whether peripheral miRNAs reflect changes in the brain or peripheral miRNA mediate changes within the brain (Maffioletti et al. 2016; Wan et al. 2015); (4) different quantification techniques’ limitations; (5) small and heterogeneous sample sizes (Yuan et al. 2018); (6) lack of studies comparing psychiatric disorders; (7) potential contamination with blood cells’ miRNAs when analyzing whole blood samples; (8) lack of genome-wide screening studies (Cairns 2015); (9) different clinical scales (Yuan et al. 2018); (10) lack of measurement of individual mature miRNA sequences.
As we mentioned earlier, chronic stress and subsequent HPA-axis upregulation contribute to the development of mental disorders and as it was shown miRNAs are frequently altered during stress conditions and potentially could be one of the molecules leading to the disease progression (Issler and Chen 2015; Luoni and Riva 2016; Zucchi et al. 2013). Although other factors (nicotine, alcohol, illicit drugs, etc.) were shown to alter miRNA expression (Kaur et al. 2012), information regarding association and psychiatric disorders is insufficient. This is an important area that requires further research.
Another obstacle in identifying psychiatry-specific miRNAs is comorbidity of somatic disorders and psychiatric disorders: MDD and ovarian cancer (Wu et al. 2018), schizophrenia and cancer (Rizos et al. 2015), etc. (Dieset et al. 2016) Although, data regarding comorbidity is inconsistent (Chou et al. 2016) and further researchers are indicated. Such comorbidity partially could be explained by pleiotropic action of miRNAs. Nevertheless, this does not mean that these diseases with 100% confidence could mimic depression in lab results of miRNAs analysis as it was performed on different specimens as well as miRNAs showed different patterns of expressions. That brings us to the idea that combination of miRNAs with structured clinical interviews could be more accurate compared to both methods used separately.
Analysis of miRNAs has its own difficulties (discordance between different techniques, dependence on sample collection and RNA extraction methods, etc.) (Gustafson et al. 2016; Tam et al. 2014; Zampetaki and Mayr 2012). Most of studies we analyzed used qRT-PCR as the main miRNA quantification method. This technique is cost-effective, has high sensitivity and specificity but unable to detect novel miRNAs and dependent on reference molecules for data normalization. To our knowledge only one study identified reference genes for qPCR analysis in neuropsychiatric disorders thus far (Liu et al. 2014). The problem of identifying novel miRNAs using qRT-PCR has resulted in very few researchers using large-scale panels in favor of small set of mostly already validated miRNAs. This could be due to the fact that despite the larger amount of post-mortem brain miRNA studies the transition of the results to peripheral specimens is troublesome because of the commonly observed inconsistency between brain-periphery expression levels of miRNA (Sun et al. 2015a, b). This statement is indirectly supported by the fact that miRNAs from CSF poorly correlated with the miRNA from plasma/serum (Gallego et al. 2018; Wan et al. 2015). There is even discordance between different peripheral specimens (plasma vs. PBMC) (Sun et al. 2015a, b).
Marco et al. in their article have shown that different mature products from the same precursor miRNA have different targeting properties and differs in their function (Marco et al. 2012). This could have a very important meaning in fundamental understanding miRNAs’ role in psychiatric disorders and therefore individual mature sequences should be identified and validated before considering any miRNA as a biomarker. All of the above together with our data showing that only 191 unique miRNAs were analyzed in 35 study leads us to consider using more sophisticated technologies like Next Generation Sequencing (NGS) in identifying both novel and already known circulating miRNAs, although, it has its own limitations which should be acknowledged.
Psychiatry has its own problems due to the body of interest: the heterogeneity of presented symptoms of individual disorder as well as huge overlap in both symptoms and underlying biology between different disorders (Doherty and Owen 2014) as we mentioned. This lead to developing of Research Domain Criteria (RDoC) project by National Institute of Mental Health in order for more adequate representation of different behavioral traits presented in psychiatric disorders with regard to underlying changes in neurobiology (Owen 2014). There is a variety of flaws in depression studies themselves: use of different clinical scales and cut-off values that make comparison even between similar studies very difficult (Yuan et al. 2018), lack of reliability and validity of MDD DSM-5 criteria diagnosis (Fried 2017). A symptom-based approach in depression with RDoC framework could potentially improve quality of not only biomarker studies in psychiatry but also could allow us to look inside fundamental neurobiology of disorders which could be clinically relevant (Fried 2015, 2017). Most of the biomarker studies we analyzed were cross-sectional, mostly patient vs. control studies, rare cross-diagnose comparison, focus on statistical significance rather than clinical relevance, lack of replication. This also prevents their use in routine psychiatric practice as diagnostic or prognostic tools, and therefore, we still rely on phenomenology-based approach (DSM) and not on objective biomarkers.
Therefore, due to huge overlaps between candidate genes and pathways and different disorders, an enormous number of targets for the single miRNA, small number of studies identifying valid targets of miRNAs in psychiatric disorders, shared symptoms in different disorders as well as heterogeneity of presentation of single disorder and other methodological limitations mentioned above makes it impossible to point out the exact mechanism of how miRNAs contribute to the pathogenesis in each particular disorder. This in term prevents their use in clinic both as diagnostic toll and potential therapy.
The limitations of our study include: (1) analysis of only three disorders; (2) exclusion of SNPs in miRNAs and genes analysis; (3) exclusion of miRNA target analysis; (4) absence of direct comparison between studies due to discordant study designs and absence of unified protocol for conducting biomarker researches in psychiatry (different isolation techniques, quantification and analyzation methods, sample sizes, inclusion/exclusion criteria, etc.); (5) exclusion of analysis of genes involved in miRNA biogenesis and function regulation.
Future Directions
All the information regarding exosomes, miRNA, psychiatric disorders and their relations as well as results obtained during our analysis have led us to several important conclusions. First, we confirmed our assumption that miRNAs in exosomes are far more suitable candidate for biomarker search based on the available literature regarding exosomes and miRNAs. This is only our hypothesis based on the available literature data as direct comparison between studies is impossible due to the aforementioned reasons. Second, we suggest that exosomes act as integration links between different pathophysiological pathways of depression mentioned in (Dean and Keshavan 2017) and extend it to psychiatric disorders in general. They act as a ‘Fed-Ex’ in our organism transferring information via biomolecules between systems that are not directly connected. Exosomes, therefore, allow this network to act as a whole as well as provide both horizontal and vertical connections between various levels of this network (e.g., immune system and CNS). This step-by-step interaction via exosomes (and most importantly miRNAs) partly explains how biological signals (e.g., stress, inflammation) are transformed into alterations in neuroplasticity which leads to changes in particular brain areas and to alterations in cognitive processes, emotional sphere, etc. That is why exosome could help us tracking down disruption in normal functioning and potentially identify more precisely what brain regions are affected in particular psychiatric disorder, how it contributes to the clinical presentation and how it correlates with other methods (e.g., MRI) which are often used as guides to where to look for altered miRNAs in post-mortem studies (Dean and Keshavan 2017; Santarelli et al. 2011). This notion is supported also by the recent review (Saeedi et al. 2019) where the authors emphasize that due to the properties of exosomes from CNS (mediators of intercellular communication, ability to cross BBB, main reservoirs of miRNAs (nervous system contributes to approximately 70% of all miRNAs), role in neuroplasticity, neurogenesis, neuroinflammation) are promising biomarker candidates as they could reflect physiologic and pathologic changes in CNS.
Our hypothesis is that exosomes could be a ‘transformer’ of biological signals into behavioral phenotypes seen in depression as well as in psychiatric disorders in general, therefore, serving as a missing link between biology and psychology (Fig. 2). They are able to act very far from their cell of origin therefore enabling effect on distant cells, tissues and organs which may account for systemic changes in mental disorders.
Fig. 2.
Schematic representation of our hypothesis regarding potential function of exosomes in pathogenesis of psychiatric disorders
Our hypothesis is supported by the facts that (1) exosomes could be secreted and captured by many cells (both immune and non-immune); (2) exosomes are able to cross BBB (or even change permeability of it); (3) exosomes are main transporters of miRNAs which were shown to regulate various pathways; (4) significant amount of miRNAs replicated in more than one study were shown to regulate BDNF expression which is one of the key players in neuroplasticity; (5) exosomes are flexible in terms of secretion and transport of biomolecules in response to various stimuli. The role of serum-derived exosomes has already been established in autism spectrum disorder: study has shown that they stimulated cultured human microglia to secrete significantly more of the pro-inflammatory IL-1β (Tsilioni and Theoharides 2018). This in concordance with other studies showing role of exosomes in neuroinflammation which in turn associated with psychiatric disorders.
All of the information above shows an intriguing potential of exosomes in both clinical and fundamental psychiatry.
Conclusions
The field of biomarker research in psychiatry grows rapidly. Nearly two hundred significantly differentially expressed miRNAs were identified in 35 studies of three major psychiatric disorders. But closer look shows that this increase was in expense of quality. Despite the availability of new technologies, we still show high rates of discordance and measure already validated miRNAs thus repeating the errors of previous studies. All these limitations and mistakes should be acknowledged and corrected. Our position is that exosomes could help in overcoming them thus providing deeper understanding the molecular biology of psychiatric disorders and eventually physiological explanation of psychological changes which can become an objective back-up for clinical decisions. We also demonstrated that diagnostic potential of miRNAs in psychiatry is not their strongest side and probably emphasis should be made on risk assessment, disease severity evaluation and treatment response but before that large-scale, prospective, cross-disorder, multi-centered studies are warranted.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author Contributions
Conceptualization, SKG and AAY; Methodology, SKG and AAY; Investigation SKG; Writing—Original Draft SKG and AAY; Writing—Review & Editing, SKG, TAD and AAY; Funding Acquisition, TAD; Resources, ABG and NVG; Supervision, ABG and NVG; Project administration, ABG and NVG.
Funding
This study was supported by the Russian Foundation for Basic Research, Project No. 17-04-01079-a.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abels ER, Breakefield XO (2016) Introduction to extracellular vesicles: biogenesis, RNA cargo selection, content, release, and uptake. Cell Mol Neurobiol 36(3):301–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksoy-Aksel A, Zampa F, Schratt G (2014) MicroRNAs and synaptic plasticity: a mutual relationship. Philos Trans R Soc Lond B Biol Sci 369(1652):20130515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alacam H, Akgun S, Akca H, Ozturk O, Kabukcu BB, Herken H (2016) Mir-181b-5p, Mir-195-5p and Mir-301a-3p are related with treatment resistance in schizophrenia. Psychiatry Res 245:200–206 [DOI] [PubMed] [Google Scholar]
- Alural B, Genc S, Haggarty SJ (2017) ‘Diagnostic and therapeutic potential of microRNAs in neuropsychiatric disorders: past, Present, and Future. Prog Neuropsychopharmacol Biol Psychiatry 73:87–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai M, Zhu X, Zhang Y, Zhang S, Zhang L, Xue L, Yi J, Yao S, Zhang X (2012) Abnormal hippocampal bdnf and Mir-16 expression is associated with depression-like behaviors induced by stress during early life. PLoS ONE 7(10):e46921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banigan MG, Kao PF, Kozubek JA, Winslow AR, Medina J, Costa J, Schmitt A, Schneider A, Cabral H, Cagsal-Getkin O, Vanderburg CR, Delalle I (2013) Differential expression of exosomal microRNAs in prefrontal cortices of schizophrenia and bipolar disorder patients. PLoS ONE 8(1):e48814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116(2):281–297 [DOI] [PubMed] [Google Scholar]
- Bavamian S, Mellios N, Lalonde J, Fass DM, Wang J, Sheridan SD, Madison JM, Zhou F, Rueckert EH, Barker D, Perlis RH, Sur M, Haggarty SJ (2015) Dysregulation of Mir-34a links neuronal development to genetic risk factors for bipolar disorder. Mol Psychiatry 20(5):573–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlim MT, Van den Eynde F, Jeff Daskalakis Z (2013) Clinically meaningful efficacy and acceptability of low-frequency repetitive transcranial magnetic stimulation (Rtms) for treating primary major depression: a meta-analysis of randomized, double-blind and sham-controlled trials. Neuropsychopharmacology 38(4):543–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge NJ, Tooney PA, Carroll AP, Gardiner E, Bowden N, Scott RJ, Tran N, Dedova I, Cairns MJ (2008) Dysregulation of miRNA 181b in the temporal cortex in schizophrenia. Hum Mol Genet 17(8):1156–1168 [DOI] [PubMed] [Google Scholar]
- Beveridge NJ, Gardiner E, Carroll AP, Tooney PA, Cairns MJ (2010) Schizophrenia is associated with an increase in cortical microRNA biogenesis. Mol Psychiatry 15(12):1176–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocchio-Chiavetto L, Maffioletti E, Bettinsoli P, Giovannini C, Bignotti S, Tardito D, Corrada D, Milanesi L, Gennarelli M (2013) Blood microRNA changes in depressed patients during antidepressant treatment. Eur Neuropsychopharmacol 23(7):602–611 [DOI] [PubMed] [Google Scholar]
- Boiko A, Losenkov I, Levchuk L, Simutkin G, Bokhan N, Bosker F, Wilffert B, Loonen A, Ivanova S (2017) Biomarkers of Depressive disorders: a multiplex analysis of blood serum. Eur Psychiatry 41:S524 [Google Scholar]
- Brett J, Renault V, Rafalski V, Webb A, Brunet A (2011) The MicroRNA cluster Mir-106b similar to 25 regulates adult neural stem/progenitor cell proliferation and neuronal differentiation. Aging Us 3(2):108–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brites D, FeRNAndes A (2015) Neuroinflammation and depression: microglia activation, extracellular microvesicles and microRNA dysregulation. Front Cell Neurosci 9:476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler A, Webb W, Lubin F (2016) Regulatory RNAs and control of epigenetic mechanisms: expectations for cognition and cognitive dysfunction. Epigenomics 8(1):135–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns M (2015) Circulating MiRNA biomarkers for schizophrenia? Am J Psychiatry 172(11):1059–1061 [DOI] [PubMed] [Google Scholar]
- Camkurt M, Acar S, Coskun S, Gunes M, Gunes S, Yilmaz M, Gorur A, Tamer L (2015) Comparison of plasma microRNA levels in drug naive, first episode depressed patients and healthy controls. J Psychiatr Res 69:67–71 [DOI] [PubMed] [Google Scholar]
- Camkurt M, Karababa F, Erdal M, Bayazit H, Kandemir S, Ay M, Kandemir H, Ay O, Cicek E, Selek S, Tasdelen B (2016) Investigation of dysregulation of several microRNAs in peripheral blood of schizophrenia patients. Clin Psychopharmacol Neurosci 14(3):256–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitano F, Camon J, Licursi V, Ferretti V, Maggi L, Scianni M, Del Vecchio G, Rinaldi A, Mannironi C, Limatola C, Presutti C, Mele A (2017) MicroRNA-335-5p modulates spatial memory and hippocampal synaptic plasticity. Neurobiol Learn Mem 139:63–68 [DOI] [PubMed] [Google Scholar]
- Caputo V, Sinibaldi L, Fiorentino A, Parisi C, Catalanotto C, Pasini A, Cogoni C, Pizzuti A (2011) Brain derived neurotrophic factor (Bdnf) expression is regulated by MicroRNAs Mir-26a and Mir-26b allele-specific binding. PLoS ONE 6(12):e28656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho A, Kohler C, Brunoni A, Miskowiak K, Herrmann N, Lanctot K, Hyphantis T, Quevedo J, FeRNAndes B, Berk M (2016) Bias in peripheral depression biomarkers. Psychother Psychosom 85(2):81–90 [DOI] [PubMed] [Google Scholar]
- Chakrabarti M, Ray S, Eyster K (2016) Experimental procedures for demonstration of microRNA mediated enhancement of functional neuroprotective effects of estrogen receptor agonists. Estrogen Recept 1366:359–372 [DOI] [PubMed] [Google Scholar]
- Chandrasekar V, Dreyer J (2011) Regulation of Mir-124, Let-7d, and Mir-181a in the accumbens affects the expression, extinction, and reinstatement of cocaine-induced conditioned place preference. Neuropsychopharmacology 36(6):1149–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Zhou Y, Wang J, Yan Y, Peng L, Qiu W (2018) Dysregulated microRNA involvement in multiple sclerosis by induction of T helper 17 cell differentiation. Front Immunol 9:1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Sharples RA, Scicluna BJ, Hill AF (2014) Exosomes provide a protective and enriched source of MiRNA for biomarker profiling compared to intracellular and cell-free blood. J Extracell Vesicles 3:23743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevillet J, Kang Q, Ruf I, Briggs H, Vojtech L, Hughes S, Cheng H, Arroyo J, Meredith E, Gallichotte E, Pogosova-Agadjanyan E, Morrissey C, Stirewalt D, Hladik F, Yu E, Higano C, Tewari M (2014) Quantitative and stoichiometric analysis of the microRNA content of exosomes. Proc Natl Acad Sci USA 111(41):14888–14893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S, Pang K, Kim J, Ryu J, Kang H, Liu Z, Kim W, Sun W, Kim H, Han K (2015) Post-transcriptional regulation of Shank3 expression by microRNAs related to multiple neuropsychiatric disorders. Mol Brain 8:74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Kao P, Itriago E, Zhan Y, Kozubek J, Hoss A, Banigan M, Vanderburg C, Rezvani A, Latourelle J, Cabral H, Delalle I (2017) Mir-149 and Mir-29c as candidates for bipolar disorder biomarkers. Am J Med Genet B 174(3):315–323 [DOI] [PubMed] [Google Scholar]
- Chou FH, Tsai KY, Wu HC, Shen SP (2016) Cancer in patients with schizophrenia: what is the next step? Psychiatry Clin Neurosci 70(11):473–488 [DOI] [PubMed] [Google Scholar]
- Cirnigliaro M, Barbagallo C, Gulisano M, Domini C, Barone R, Barbagallo D, Ragusa M, Di Pietro C, Rizzo R, Purrello M (2017) Expression and regulatory network analysis of Mir-140-3p, a new potential serum biomarker for autism spectrum disorder. Front Mol Neurosci 10:250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton A, Buschmann D, Byrd J, Carter D, Cheng L, Compton C, Daaboul G, Devitt A, Falcon-Perez J, Gardiner C, Gustafson D, Harrison P, Helmbrecht C, Hendrix A, Hill A, Hoffman A, Jones J, Kalluri R, Kang J, Kirchner B, Lasser C, Lawson C, Lenassi M, Levin C, Llorente A, Martens-Uzunova E, Moller A, Musante L, Ochiya T, Pink R, Tahara H, Wauben M, Webber J, Welsh J, Witwer K, Yin H, Nieuwland R (2018) Summary of the Isev Workshop on extracellular vesicles as disease biomarkers, held in Birmingham, UK, during December 2017. J Extracell Vesicles 7:1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo M, Raposo G, Thery C, Schekman R, Lehmann R (2014) Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 30:255–289 [DOI] [PubMed] [Google Scholar]
- Cox M, Cairns M, Gandhi K, Carroll A, Moscovis S, Stewart G, Broadley S, Scott R, Booth D, Lechner-Scott J, Genetic AMS (2010) MicroRNAs Mir-17 and Mir-20a inhibit T cell activation genes and are under-expressed in ms whole blood. PLoS ONE 5:8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadkhah T, Rahimi-Aliabadi S, Jamshidi J, Ghaedi H, Taghavi S, Shokraeian P, Akhavan-Niaki H, Tafakhori A, Ohadi M, Darvish H (2017) A genetic variant in MiRNA binding site of glutamate receptor 4, metabotropic (Grm4) is associated with increased risk of major depressive disorder. J Affect Disord 208:218–222 [DOI] [PubMed] [Google Scholar]
- Dean J, Keshavan M (2017) The neurobiology of depression: an integrated view. Asian J Psychiatry 27:101–111 [DOI] [PubMed] [Google Scholar]
- Dieset I, Andreassen OA, Haukvik UK (2016) Somatic comorbidity in schizophrenia: some possible biological mechanisms across the life span. Schizophr Bull 42(6):1316–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty J, Owen M (2014) Genomic insights into the overlap between psychiatric disorders: implications for research and clinical practice. Genome Med 6:29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedi Y (2011) Evidence demonstrating role of microRNAs in the etiopathology of major depression. J Chem Neuroanat 42(2):142–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedi Y (2014) Emerging role of microRNAs in major depressive disorder: diagnosis and therapeutic implications. Dialogues Clin Neurosci 16(1):43–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enatescu VR, Papava I, Enatescu I, Antonescu M, Anghel A, Seclaman E, Sirbu IO, Marian C (2016) Circulating plasma micro RNAs in patients with major depressive disorder treated with antidepressants: a pilot study. Psychiatry Investig 13(5):549–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbri M, Paone A, Calore F, Galli R, Gaudio E, Santhanam R, Lovat F, Fadda P, Mao C, Nuovo GJ, Zanesi N, Crawford M, Ozer GH, Wernicke D, Alder H, Caligiuri MA, Nana-Sinkam P, Perrotti D, Croce CM (2012) MicroRNAs bind to toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci USA 109(31):E2110–E2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, Qiu Q, Zhang S, Sun L, Li G, Xiao S, Li X (2018) Changes in MiRNA-132 and Mir-124 levels in non-treated and citalopram-treated patients with depression. J Affect Disord 227:745–751 [DOI] [PubMed] [Google Scholar]
- FeRNAndes BS, Molendijk ML, Köhler CA, Soares JC, Leite CM, Machado-Vieira R, Ribeiro TL, Silva JC, Sales PM, Quevedo J, Oertel-Knöchel V, Vieta E, González-Pinto A, Berk M, Carvalho AF (2015a) Peripheral brain-derived neurotrophic factor (Bdnf) as a biomarker in bipolar disorder: a meta-analysis of 52 studies. BMC Med 13:289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FeRNAndes BS, Steiner J, Berk M, Molendijk ML, Gonzalez-Pinto A, Turck CW, Nardin P, Gonçalves CA (2015b) Peripheral brain-derived neurotrophic factor in schizophrenia and the role of antipsychotics: meta-analysis and implications. Mol Psychiatry 20(9):1108–1119 [DOI] [PubMed] [Google Scholar]
- Ferrari AJ, Stockings E, Khoo JP, Erskine HE, Degenhardt L, Vos T, Whiteford HA (2016) The prevalence and burden of bipolar disorder: findings from the global burden of disease study 2013. Bipolar Disord 18(5):440–450 [DOI] [PubMed] [Google Scholar]
- Fiandaca MS, Kapogiannis D, Mapstone M, Boxer A, Eitan E, Schwartz JB, Abner EL, Petersen RC, Federoff HJ, Miller BL, Goetzl EJ (2015) Identification of preclinical Alzheimer’s disease by a profile of pathogenic proteins in neurally derived blood exosomes: a case-control study. Alzheimers Dementia 11(6):600–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueredo DES, Barbosa MR, Gitaí DL, de Andrade TG (2013) Predicted microRNAs for mammalian circadian rhythms. J Biol Rhythms 28(2):107–116 [DOI] [PubMed] [Google Scholar]
- Fiori LM, Lopez JP, Richard-Devantoy S, Berlim M, Chachamovich E, Jollant F, Foster J, Rotzinger S, Kennedy SH, Turecki G (2017) Investigation of Mir-1202, Mir-135a, and Mir-16 in major depressive disorder and antidepressant response. Int J Neuropsychopharmacol 20(8):619–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried EI (2015) ‘Problematic assumptions have slowed down depression research: why symptoms, not syndromes are the way forward. Front Psychol 6:309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried EI (2017) Moving forward: how depression heterogeneity hinders progress in treatment and research. Expert Rev Neurother 17(5):423–425 [DOI] [PubMed] [Google Scholar]
- Fries GR, Carvalho AF, Quevedo J (2018) The mirnome of bipolar disorder. J Affect Disord 233:110–116 [DOI] [PubMed] [Google Scholar]
- Gallego J, Alsop E, Lencz T, Van Keuren-Jensen K, Malhotra A (2018) Differential expression of microRNAs in cerebrospinal fluid and plasma samples in schizophrenia. Schizophr Bull 44:S221–S222 [Google Scholar]
- Gao Q, Zhou L, Yang S, Cao J (2016) A novel role of microRNA 17-5p in the modulation of circadian rhythm. Sci Rep 6:30070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbett K, Vereczkei A, Kaman S, Wang L, Korade Z, Shelton R, Mirnics K (2015) Fibroblasts from patients with major depressive disorder show distinct transcriptional response to metabolic stressors. Transl Psychiatry 5:e523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geaghan M, Cairns M (2015) MicroRNA and posttranscriptional dysregulation in psychiatry. Biol Psychiat 78(4):231–239 [DOI] [PubMed] [Google Scholar]
- Gheysarzadeh A, Sadeghifard N, Afraidooni L, Pooyan F, Mofid M, Valadbeigi H, Bakhtiari H, Keikhavani S (2018) Serum-based microRNA biomarkers for major depression: Mir-16, Mir-135a, and Ma-1202. J Res Med Sci 23 [DOI] [PMC free article] [PubMed]
- Girardi P, Pompili M, Innamorati M, Mancini M, Serafini G, Mazzarini L, Del Casale A, Tatarelli R, Baldessarini RJ (2009) Duloxetine in acute major depression: review of comparisons to placebo and standard antidepressants using dissimilar methods. Hum Psychopharmacol 24(3):177–190 [DOI] [PubMed] [Google Scholar]
- Goldie B, Dun M, Lin M, Smith N, Verrills N, Dayas C, Cairns M (2014) Activity-associated MiRNA are packaged in Map1b-enriched exosomes released from depolarized neurons. Nucleic Acids Res 42(14):9195–9208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J, Fan K, Zhang J, Chen H, Wang S (2018) Suppression of MicroRNA-130b Inhibits glioma cell proliferation and invasion, and induces apoptosis by pten/akt signaling. Int J Mol Med 41(1):284–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo A, Sun J, Jia P, Zhao Z (2010) A novel microRNA and transcription factor mediated regulatory network in schizophrenia. BMC Syst Biol 4:10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gururajan A, Naughton M, Scott K, O’Connor R, Moloney G, Clarke G, Dowling J, Walsh A, Ismail F, Shorten G, Scott L, McLoughlin D, Cryan J, Dinan T (2016) MicroRNAs as biomarkers for major depression: a role for Let-7b and Let-7c. Transl Psychiatry 6:e862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson D, Tyryshkin K, Renwick N (2016) MicroRNA-guided diagnostics in clinical samples. Best Pract Res Clin Endocrinol Metab 30(5):563–575 [DOI] [PubMed] [Google Scholar]
- Ha M, Kim V (2014) Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol 15(8):509–524 [DOI] [PubMed] [Google Scholar]
- He K, Guo C, He L, Shi Y (2017) MiRNAs of peripheral blood as the biomarker of schizophrenia. Hereditas 155:1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hombach S, Kretz M (2016) Non-coding RNAs: classification, biology and functioning. Adv Exp Med Biol 937:3–17 [DOI] [PubMed] [Google Scholar]
- Hsu P, Xu B, Mukai J, Karayiorgou M, Gogos J (2015) The Bdnf val66met variant affects gene expression through Mir-146b. Neurobiol Dis 77:228–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issler O, Chen A (2015) Determining the role of microRNAs in psychiatric disorders. Nat Rev Neurosci 16(4):201–212 [DOI] [PubMed] [Google Scholar]
- Issler O, Haramati S, Paul E, Maeno H, Navon I, Zwang R, Gil S, Mayberg H, Dunlop B, Menke A, Awatramani R, Binder E, Deneris E, Lowry C, Chen A (2014) MicroRNA 135 is essential for chronic stress resiliency, antidepressant efficacy, and intact serotonergic activity. Neuron 83(2):344–360 [DOI] [PubMed] [Google Scholar]
- Ivan M, Huang X, Koumenis C, Hammond E, Giaccia A (2014) Mir-210: fine-tuning the hypoxic response. Tumor Microenviron Cell Stress 772:205–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janas A, Sapon K, Janas T, Stowell M (2016) Exosomes and other extracellular vesicles in neural cells and neurodegenerative diseases. Biochim Biophys Acta-Biomembr 1858(6):1139–1151 [DOI] [PubMed] [Google Scholar]
- Jiang H, Chen S, Li C, Lu N, Yue Y, Yin Y, Zhang Y, Zhi X, Zhang D, Yuan Y (2017) The serum protein levels of the tpa-bdnf pathway are implicated in depression and antidepressant treatment. Transl Psychiatry 7:e1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Kim S, Liu X, Zhang H, Zhang C, Seo J, Kim Y, Sun T (2016) Mir-17-92 cluster regulates adult hippocampal neurogenesis, anxiety, and depression. Cell Rep 16(6):1653–1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatsoreos I (2014) Links between circadian rhythms and psychiatric disease. Front Behav Neurosci 8:162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur P, Armugam A, Jeyaseelan K (2012) MicroRNAs in neurotoxicity. J Toxicol 2012:870150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kichukova TM, Popov NT, Ivanov HY, Vachev TI (2015) Circulating microRNAs as a novel class of potential diagnostic biomarkers in neuropsychiatric disorders. Folia Med 57(3–4):159–172 [DOI] [PubMed] [Google Scholar]
- Kolshus E, Dalton VS, Ryan KM, McLoughlin DM (2014) When less is more-microRNAs and psychiatric disorders. Acta Psychiatr Scand 129(4):241–256 [DOI] [PubMed] [Google Scholar]
- Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, Dingli F, Loew D, Tkach M, Théry C (2016) Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci USA 113(8):E968–E977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang WH, Dong ZQ, Tian LT, Li J (2018) MicroRNA-451a, MicroRNA-34a-5p, and MicroRNA-221-3p as predictors of response to antidepressant treatment. Braz J Med Biol Res 51(7):e7212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwano N, Kato T, Mitsuhashi M, Sato-Kasai M, Shimokawa N, Hayakawa K, Ohgidani M, Sagata N, Kubo H, Sakurai T, Kanba S (2018) Neuron-related blood inflammatory markers as an objective evaluation tool for major depressive disorder: an exploratory pilot case-control study. J Affect Disord 240:88–98 [DOI] [PubMed] [Google Scholar]
- Labouesse M, Polesel M, Clementi E, Muller F, Markkanen E, Mouttet F, Cattaneo A, Richetto J (2018) MicroRNA expression profiling in the prefrontal cortex: putative mechanisms for the cognitive effects of adolescent high fat feeding. Scientific Reports 8:8344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafourcade C, Ramirez J, Luarte A, FeRNAndez A, Wyneken U (2016) MiRNAs in astrocyte-derived exosomes as possible mediators of neuronal plasticity. J Exp Neurosci 10:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazaro-Ibanez E, Sanz-Garcia A, Visakorpi T, Escobedo-Lucea C, Siljander P, Ayuso-Sacido A, Yliperttula M (2014) Different Gdna content in the subpopulations of prostate cancer extracellular vesicles: apoptotic bodies, microvesicles, and exosomes. Prostate 74(14):1379–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Xu M, Gao Z, Wang Y, Yue Z, Zhang Y, Li X, Zhang C, Xie S, Wang P (2013) Alterations of Serum levels of Bdnf-related MiRNAs in patients with depression. PLoS ONE 8:5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Zeringer E, Barta T, Schageman J, Cheng A, Vlassov A (2014) Analysis of the RNA content of the exosomes derived from blood serum and urine and its potential as biomarkers. Philos Trans R Soc B 369:1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Meng H, Cao W, Qiu T (2015) Lmir-335 Is involved in major depression disorder and antidepressant treatment through targeting Grm4. Neurosci Lett 606:167–172 [DOI] [PubMed] [Google Scholar]
- Li Y, Li S, Yan J, Wang D, Yin R, Zhao L, Zhu Y, Zhu X (2016a) Mir-182 (MicroRNA-182) suppression in the Hippocampus evokes antidepressant-like effects in rats. Prog Neuropsychopharmacol Biol Psychiatry 65:96–103 [DOI] [PubMed] [Google Scholar]
- Li Z, Li Y, Wang N, Yang L, Zhao W, Zeng X (2016b) Mir-130b Targets Nkd2 and regulates the wnt signaling to promote proliferation and inhibit apoptosis in osteosarcoma cells. Biochem Biophys Res Commun 471(4):479–485 [DOI] [PubMed] [Google Scholar]
- Li N, Miao Y, Shan Y, Liu B, Li Y, Zhao L, Jia L (2017) Mir-106b and Mir-93 regulate cell progression by suppression of Pten Via Pi3k/Akt pathway in breast cancer. Cell Death Dis 8:e2796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Yang H, Ma J, Luo S, Chen S, Gu Q (2018a) MicroRNA-30e regulates neuroinflammation in Mptp model of Parkinson’s disease by targeting Nlrp3. Hum Cell 31(2):106–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Wang L, Li X, Chen X, Lu B, Cheng L, Yan C, Xu Y (2018b) Total salvianolic acid balances brain functional network topology in rat hippocampi overexpressing Mir-30e. Front Neurosci 12:448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao X, Mao W, Wang Q, Yang G, Wu W, Shao S (2016) MicroRNA-24 inhibits serotonin reuptake transporter expression and aggravates irritable bowel syndrome. Biochem Biophys Res Commun 469(2):288–293 [DOI] [PubMed] [Google Scholar]
- Liguori M, Nuzziello N, Licciulli F, Consiglio A, Simone M, Viterbo R, Creanza T, Ancona N, Tortorella C, Margari L, Grillo G, Giordano P, Liuni S, Trojano M (2018) Combined MicroRNA and MRNA expression analysis in pediatric multiple sclerosis: an integrated approach to uncover novel pathogenic mechanisms of the disease. Hum Mol Genet 27(1):66–79 [DOI] [PubMed] [Google Scholar]
- Lim C, Zainal N, Kanagasundram S, Zain S, Mohamed Z (2016) Preliminary examination of MicroRNA expression profiling in bipolar disorder I patients during antipsychotic treatment. Am J Med Genet B 171(6):867–874 [DOI] [PubMed] [Google Scholar]
- Liu Y, Lu Q (2015) Extracellular vesicle microRNAs: biomarker discovery in various diseases based on Rt-QPCR. Biomark Med 9(8):791–805 [DOI] [PubMed] [Google Scholar]
- Liu J, Su B (2018) Integrated analysis supports Atxn1 as a schizophrenia risk gene. Schizophr Res 195:298–305 [DOI] [PubMed] [Google Scholar]
- Liu S, Yuan Y, Guan L, Wei H, Cheng Z, Han X, Yang L, Pu C, Yang F, Lu Z, Deng H, Zhao J, Yu X (2013) MiRNA-365 and MiRNA-520c-3p respond to risperidone treatment in first-episode schizophrenia after a 1 Year remission. Chin Med J 126(14):2676–2680 [PubMed] [Google Scholar]
- Liu X, Zhang L, Cheng K, Wang X, Ren G, Xie P (2014) Identification of suitable plasma-based reference genes for miRNAome analysis of major depressive disorder. J Affect Disord 163:133–139 [DOI] [PubMed] [Google Scholar]
- Liu X, Yan S, Pei C, Cui Y (2015) Decreased MicroRNA-132 and its function in human non-small cell lung cancer. Mol Med Rep 11(5):3601–3608 [DOI] [PubMed] [Google Scholar]
- Liu Y, Yang X, Zhao L, Zhang J, Li T, Ma X (2016) Increased Mir-132 level is associated with visual memory dysfunction in patients with depression. Neuropsychiatr Dis Treat 12:2905–2911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Liu J, Wang M, Zhang Y, Li L (2017) From serotonin to neuroplasticity: evolvement of theories for major depressive disorder. Front Cell Neurosci 11:305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Zhang L, Li H (2018) New Insights: microRNA function in CNS development and psychiatric diseases. Curr Pharmacol Rep 4(2):132–144 [Google Scholar]
- Lopez J, Lim R, Cruceanu C, Crapper L, Fasano C, Labonte B, Maussion G, Yang J, Yerko V, Vigneault E, El Mestikawy S, Mechawar N, Pavlidis P, Turecki G (2014) Mir-1202 is a primate-specific and brain-enriched microRNA involved in major depression and antidepressant treatment. Nat Med 20(7):764–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez J, Fiori L, Cruceanu C, Lin R, Labonte B, Cates H, Heller E, Vialou V, Ku S, Gerald C, Han M, Foster J, Frey B, Soares C, Muller D, Farzan F, Leri F, MacQueen G, Feilotter H, Tyryshkin K, Evans K, Giacobbe P, Blier P, Lam R, Milev R, Parikh S, Rotzinger S, Strother S, Lewis C, Aitchison K, Wittenberg G, Mechawar N, Nestler E, Uher R, Kennedy S, Turecki G (2017) MicroRNAs 146a/B-5 and 425-3p and 24-3p Are Markers of antidepressant response and regulate Mapk/Wnt-system genes. Nat Commun 8:15497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Wei J, Feng Z, Chen Z, Wang Y, Huang Y, Fang Y, Liang Y, Cen J, Pan Y, Liao B, Chen W, Chen W, Luo J (2017) Mir-106b-5p promotes renal cell carcinoma aggressiveness and stem-cell-like phenotype by activating Wnt/Beta-Catenin signalling. Oncotarget 8(13):21461–21471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luoni A, Riva MA (2016) MicroRNAs and psychiatric disorders: from aetiology to treatment. Pharmacol Ther 167:13–27 [DOI] [PubMed] [Google Scholar]
- Ma J, Shang S, Wang J, Zhang T, Nie F, Song X, Zhao H, Zhu C, Zhang R, Hao D (2018) Identification of Mir-22-3p, Mir-92a-3p, and Mir-137 in peripheral blood as biomarker for schizophrenia. Psychiatry Res 265:70–76 [DOI] [PubMed] [Google Scholar]
- Maffioletti E, Tardito D, Gennarelli M, Bocchio-Chiavetto L (2014) Micro spies from the brain to the periphery: new clues from studies on MicroRNAs in neuropsychiatric disorders. Front Cell Neurosci 8:75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffioletti E, Cattaneo A, Rosso G, Maina G, Maj C, Gennarelli M, Tardito D, Bocchio-Chiavetto L (2016) Peripheral whole blood microRNA alterations in major depression and bipolar disorder. J Affect Disord 200:250–258 [DOI] [PubMed] [Google Scholar]
- Mannironi C, Biundo A, Rajendran S, De Vito F, Saba L, Caioli S, Zona C, Ciotti T, Caristi S, Perlas E, Del Vecchio G, Bozzoni I, Rinaldi A, Mele A, Presutti C (2018) Mir-135a regulates synaptic transmission and anxiety-like behavior in amygdala. Mol Neurobiol 55(4):3301–3315 [DOI] [PubMed] [Google Scholar]
- Marco A, Macpherson JI, Ronshaugen M, Griffiths-Jones S (2012) MicroRNAs from the same precursor have different targeting properties. Silence 3(1):8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques F, Eikelis N, Bayles R, Lambert E, Straznicky N, Hering D, Esler M, Head G, Barton D, Schlaich M, Lambert G (2017) A polymorphism in the norepinephrine transporter gene is associated with affective and cardiovascular disease through a microRNA mechanism. Mol Psychiatry 22(1):134–141 [DOI] [PubMed] [Google Scholar]
- Mateescu B, Kowal E, van Balkom B, Bartel S, Bhattacharyya S, Buzas E, Buck A, de Candia P, Chow F, Das S, Driedonks T, FeRNAndez-Messina L, Haderk F, Hill A, Jones J, Van Keuren-Jensen K, Lai C, Lasser C, di Liegro I, Lunavat T, Lorenowicz M, Maas S, Mager I, Mittelbrunn M, Momma S, Mukherjee K, Nawaz M, Pegtel D, Pfaffl M, Schiffelers R, Tahara H, Thery C, Tosar J, Wauben M, Witwer K, Nolte-’T Hoen E (2017) Obstacles and opportunities in the functional analysis of extracellular vesicle RNA: an Isev position paper. J Extracell Vesicles 6:1286095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes-Silva A, Pereira K, Tolentino-Araujo G, Nicolau E, Silva-Ferreira C, Teixeira A, Diniz B (2016) Shared biologic pathways between Alzheimer disease and major depression: a systematic review of microRNA expression studies. Am J Geriatr Psychiatry 24(10):903–912 [DOI] [PubMed] [Google Scholar]
- Miller A, Raison C (2016) The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol 16(1):22–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S, Dell’Osso B, Ketter T (2014) The prevalence and burden of bipolar depression. J Affect Disord 169:S3–S11 [DOI] [PubMed] [Google Scholar]
- Mitchell A, Vaze A, Rao S (2009) Clinical diagnosis of depression in primary care: a meta-analysis. Lancet 374(9690):609–619 [DOI] [PubMed] [Google Scholar]
- Mohr A, Mott J (2015) Overview of microRNA biology. Semin Liver Dis 35(1):3–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojtabai R (2013) Clinician-identified depression in community settings: concordance with structured-interview diagnoses. Psychother Psychosom 82(3):161–169 [DOI] [PubMed] [Google Scholar]
- Mojtabai R, Olfson M, Han B (2016) National trends in the prevalence and treatment of depression in adolescents and young adults. Pediatrics 138:6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molendijk M, Bus B, Spinhoven P, Penninx B, Prickaerts J, Voshaar R, Elzinga B (2012) Gender specific associations of serum levels of brain-derived neurotrophic factor in anxiety. World J Biol Psychiatry 13(7):535–543 [DOI] [PubMed] [Google Scholar]
- Molendijk M, Spinhoven P, Polak M, Bus B, Penninx B, Elzinga B (2014) Serum Bdnf concentrations as peripheral manifestations of depression: evidence from a systematic review and meta-analyses on 179 associations (N = 9484). Mol Psychiatry 19(7):791–800 [DOI] [PubMed] [Google Scholar]
- Moreno-Kustner B, Martin C, Pastor L (2018) Prevalence of psychotic disorders and its association with methodological issues. A systematic review and meta-analyses. PLoS ONE 13:4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narahari A, Hussain M, Sreeram V (2017) MicroRNAs as biomarkers for psychiatric conditions: a review of current research. Innov Clin Neurosci 14(1–2):53–55 [PMC free article] [PubMed] [Google Scholar]
- Niciu MJ, Kelmendi B, Sanacora G (2012) Overview of glutamatergic neurotransmission in the nervous system. Pharmacol Biochem Behav 100(4):656–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak JS, Michlewski G (2013) MiRNAs in development and pathogenesis of the nervous system. Biochem Soc Trans 41(4):815–820 [DOI] [PubMed] [Google Scholar]
- Organization WH (2017) Depression and other common mental disorders: global health estimates. World Health Organization, Geneva [Google Scholar]
- Owen MJ (2014) New approaches to psychiatric diagnostic classification. Neuron 84(3):564–571 [DOI] [PubMed] [Google Scholar]
- Palazidou E (2012) The neurobiology of depression. Br Med Bull 101(1):127–145 [DOI] [PubMed] [Google Scholar]
- Park S-C, Kim Y-K (2018) Depression in Dsm-5: changes, controversies, and future directions. In: Kim Y-K (ed) Understanding depression. Clinical manifestations, diagnosis and treatment, vol 2. Springer, Singapore [Google Scholar]
- Pelletier L, O’Donnell S, Dykxhoorn J, McRae L, Patten S (2017) Under-diagnosis of mood disorders in Canada. Epidemiol Psychiatr Sci 26(4):414–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichler S, Gu W, Hartl D, Gasparoni G, Leidinger P, Keller A, Meese E, Mayhaus M, Hampel H, Riemenschneider M (2017) The mirnome of Alzheimer’s disease: consistent downregulation of the Mir-132/212 cluster. Neurobiol Aging 50:167.e1–167.e10 [DOI] [PubMed] [Google Scholar]
- Pinto JV, Moulin TC, Amaral OB (2017) On the transdiagnostic nature of peripheral biomarkers in major psychiatric disorders: a systematic review. Neurosci Biobehav Rev 83:97–108 [DOI] [PubMed] [Google Scholar]
- Polyakova M, Stuke K, Schuemberg K, Mueller K, Schoenknecht P, Schroeter ML (2015) Bdnf as a biomarker for successful treatment of mood disorders: a systematic & quantitative meta-analysis. J Affect Disord 174:432–440 [DOI] [PubMed] [Google Scholar]
- Pompili M, Serafini G, Innamorati M, Venturini P, Fusar-Poli P, Sher L, Amore M, Girardi P (2013) Agomelatine, a novel intriguing antidepressant option enhancing neuroplasticity: a critical review. World J Biol Psychiatry 14(6):412–431 [DOI] [PubMed] [Google Scholar]
- Prada I, Gabrielli M, Turola E, Iorio A, D’Arrigo G, Parolisi R, De Luca M, Pacifici M, Bastoni M, Lombardi M, Legname G, Cojoc D, Buffo A, Furlan R, Peruzzi F, Verderio C (2018) Glia-to-neuron transfer of MiRNAs via extracellular vesicles: a new mechanism underlying inflammation-induced synaptic alterations. Acta Neuropathol 135(4):529–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prata J, Santos S, Almeida M, Coelho R, Barbosa M (2017) Bridging autism spectrum disorders and schizophrenia through inflammation and biomarkers: pre-clinical and clinical investigations. J Neuroinflammation 14:179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizos E, Siafakas N, Katsantoni E, Skourti E, Salpeas V, Rizos I, Tsoporis JN, Kastania A, Filippopoulou A, Xiros N, Margaritis D, Parker TG, Papageorgiou C, Zoumpourlis V (2015) Correction: let-7, Mir-98 and Mir-181 as biomarkers for cancer and schizophrenia. PLoS ONE 10(8):e0135863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong H, Liu T, Yang K, Yang H, Wu D, Liao C, Hong F, Yang H, Wan F, Ye X, Xu D, Zhang X, Chao C, Shen Q (2011) MicroRNA-134 plasma levels before and after treatment for bipolar mania. J Psychiatr Res 45(1):92–95 [DOI] [PubMed] [Google Scholar]
- Roy B, Dunbar M, Shelton R, Dwivedi Y (2017a) Identification of MicroRNA-124-3p as a putative epigenetic signature of major depressive disorder. Neuropsychopharmacology 42(4):864–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy B, Wang Q, Palkovits M, Faludi G, Dwivedi Y (2017b) Altered MiRNA expression network in locus coeruleus of depressed suicide subjects. Sci Rep 7:4387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeedi S, Israel S, Nagy C, Turecki G (2019) The emerging role of exosomes in mental disorders. Transl Psychiatry 9(1):122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salta E, De Strooper B (2017) MicroRNA-132: a key noncoding RNA operating in the cellular phase of Alzheimer’s disease. Faseb J 31(2):424–433 [DOI] [PubMed] [Google Scholar]
- Santarelli DM, Beveridge NJ, Tooney PA, Cairns MJ (2011) Upregulation of Dicer and microRNA expression in the dorsolateral prefrontal cortex brodmann area 46 in schizophrenia. Biol Psychiatry 69(2):180–187 [DOI] [PubMed] [Google Scholar]
- Saus E, Soria V, Escaramis G, Vivarelli F, Crespo J, Kagerbauer B, Menchon J, Urretavizcaya M, Gratacos M, Estivill X (2010) Genetic variants and abnormal processing of Pre-Mir-182, a circadian clock modulator, in major depression patients with late insomnia. Hum Mol Genet 19(20):4017–4025 [DOI] [PubMed] [Google Scholar]
- Scott H (2017) Extracellular microRNAs as messengers in the central and peripheral nervous system. Neuronal Signal, NS20170112 [DOI] [PMC free article] [PubMed]
- Serafini G, Pompili M, Belvederi Murri M, Respino M, Ghio L, Girardi P, Fitzgerald PB, Amore M (2015) The effects of repetitive transcranial magnetic stimulation on cognitive performance in treatment-resistant depression. a systematic review. Neuropsychobiology 71(3):125–139 [DOI] [PubMed] [Google Scholar]
- Sethi S (2017) The potential role of miRNAs in schizophrenia: diagnostic and therapeutic applications. EC Psychol Psychiatry 3:92–101 [Google Scholar]
- Shao Q, You F, Zhang Y, Hu L, Liu W, Liu Y, Li J, Wang S, Song M (2018) Csf Mir-16 expression and its association with Mir-16 and serotonin transporter in the raphe of a rat model of depression. J Affect Disord 238:609–614 [DOI] [PubMed] [Google Scholar]
- Shen F, Huang W, Xing B, Fang X, Feng M, Jiang C (2018) Genistein improves the major depression through suppressing the expression of Mir-221/222 by targeting connexin 43. Psychiatry Investig 15(10):919–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi W, Du J, Qi Y, Liang G, Wang T, Li S, Xie S, Zeshan B, Xiao Z (2012) Aberrant expression of serum miRNAs in schizophrenia. J Psychiatr Res 46(2):198–204 [DOI] [PubMed] [Google Scholar]
- Sodar B, Kittel A, Paloczi K, Vukman K, Osteikoetxea X, Szabo-Taylor K, Nemeth A, Sperlagh B, Baranyai T, Giricz Z, Wiener Z, Turiak L, Drahos L, Pallinger E, Vekey K, Ferdinandy P, Falus A, Buzas E (2016) Low-density lipoprotein mimics blood plasma-derived exosomes and microvesicles during isolation and detection. Sci Rep 6:24316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohel MM (2016) Extracellular/circulating microRNAs: release mechanisms, functions and challenges. Achiev Life Sci 10:175–186 [Google Scholar]
- Song H, Sun X, Zhang L, Zhao L, Guo Z, Fan H, Zhong A, Niu W, Dai Y, Zhang L, Shi Z, Liu X, Lu J (2014) A preliminary analysis of association between the down-regulation of MicroRNA-181b expression and symptomatology improvement in schizophrenia patients before and after antipsychotic treatment. J Psychiatr Res 54:134–140 [DOI] [PubMed] [Google Scholar]
- Su M, Hong J, Zhao Y, Liu S, Xue X (2015) Mecp2 controls hippocampal brain-derived neurotrophic factor expression via homeostatic interactions with microRNA-132 in rats with depression. Mol Med Rep 12(4):5399–5406 [DOI] [PubMed] [Google Scholar]
- Suliman S, Hemmings S, Seedat S (2013) Brain-derived neurotrophic factor (Bdnf) protein levels in anxiety disorders: systematic review and meta-regression analysis. S Afr J Psychiatry 19(3):124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun E, Shi Y (2015) MicroRNAs: small molecules with big roles in neurodevelopment and diseases. Exp Neurol 268:46–53 [DOI] [PubMed] [Google Scholar]
- Sun X, Lu J, Zhang L, Song H, Zhao L, Fan H, Zhong A, Niu W, Guo Z, Dai Y, Chen C, Ding Y, Zhang L (2015a) Aberrant microRNA expression in peripheral plasma and mononuclear cells as specific blood-based biomarkers in schizophrenia patients. J Clin Neurosci 22(3):570–574 [DOI] [PubMed] [Google Scholar]
- Sun X, Zhang J, Niu W, Guo W, Song H, Li H, Fan H, Zhao L, Zhong A, Dai Y, Guo Z, Zhang L, Lu J, Zhang Q (2015b) A preliminary analysis of microRNA as potential clinical biomarker for schizophrenia. Am J Med Genet B 168(3):170–178 [DOI] [PubMed] [Google Scholar]
- Tam S, de Borja R, Tsao M, McPherson J (2014) Robust global microRNA expression profiling using next-generation sequencing technologies. Lab Invest 94(3):350–358 [DOI] [PubMed] [Google Scholar]
- Tamkovich S, Tutanov O, Laktionov P (2016) Exosomes: generation, structure, transport, biological activity, and diagnostic application. Biochem Moscow Suppl Ser A 10(3):163–173 [Google Scholar]
- Tapia-Arancibia L, Rage F, Givalois L, Arancibia S (2004) Physiology of Bdnf: focus on hypothalamic function. Front Neuroendocrinol 25(2):77–107 [DOI] [PubMed] [Google Scholar]
- Thomson D, Bracken C, Goodall G (2011) Experimental strategies for microRNA target identification. Nucleic Acids Res 39(16):6845–6853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thyloth M, Singh H, Subramanian V (2016) Increasing burden of mental illnesses across the globe: current status. Indian J Soc Psychiatry 32(3):254–256 [Google Scholar]
- Tian F, Shen Y, Chen Z, Li R, Ge Q (2017) No significant difference between plasma miRNAs and plasma-derived exosomal miRNAs from healthy people. Biomed Res Int. 10.1155/2017/1304816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkach M, Thery C (2016) Communication by extracellular vesicles: where we are and where we need to go. Cell 164(6):1226–1232 [DOI] [PubMed] [Google Scholar]
- Tohda M, Mingmalairak S, Murakami Y, Matsumoto K (2010) Enhanced expression of Bcl2/adenovirus Eib 19-kDa-interacting protein 3 MRNA, a candidate for intrinsic depression-related factor, and effects of imipramine in the frontal cortex of stressed mice. Biol Pharm Bull 33(1):53–57 [DOI] [PubMed] [Google Scholar]
- Tosar J, Gambaro F, Sanguinetti J, Bonilla B, Witwer K, Cayota A (2015) Assessment of small RNA sorting into different extracellular fractions revealed by high-throughput sequencing of breast cell lines. Nucleic Acids Res 43(11):5601–5616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsilioni I, Theoharides T (2018) Extracellular vesicles are increased in the serum of children with autism spectrum disorder, contain mitochondrial dna, and stimulate human microglia to secrete Il-1 beta. J Neuroinflammation 15:239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uher R, Payne J, Pavlova B, Perlis R (2014) Major depressive disorder in Dsm-5: implications for clinical practice and research of changes from Dsm-Iv. Depress Anxiety 31(6):459–471 [DOI] [PubMed] [Google Scholar]
- Undurraga J, Baldessarini RJ (2012) randomized, placebo-controlled trials of antidepressants for acute major depression: thirty-year meta-analytic review. Neuropsychopharmacology 37(4):851–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Niel G, D’Angelo G, Raposo G (2018) Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol 19(4):213–228 [DOI] [PubMed] [Google Scholar]
- Varendi K, Matlik K, Andressoo J (2015) From MicroRNA target validation to therapy: lessons learned from studies on Bdnf. Cell Mol Life Sci 72(9):1779–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan S (2012) Posttranscriptional upregulation by MicroRNAs. Wiley Interdiscip Rev 3(3):311–330 [DOI] [PubMed] [Google Scholar]
- Vickers K, Palmisano B, Shoucri B, Shamburek R, Remaley A (2011) MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol 13(4):423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Bartheld CS, Altick AL (2011) Multivesicular bodies in neurons: distribution, protein content, and trafficking functions. Prog Neurobiol 93(3):313–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker R, Rybka J, Anderson S, Torrance H, Boxall R, Sussmann J, Porteous D, McIntosh A, Evans K (2015) Preliminary investigation of miRNA expression in individuals at high familial risk of bipolar disorder. J Psychiatr Res 62:48–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y, Liu Y, Wang X, Wu J, Liu K, Zhou J, Liu L, Zhang C (2015) Identification of differential microRNAs in cerebrospinal fluid and serum of patients with major depressive disorder. PLoS ONE 10:3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanet A, Tacheny A, Arnould T, Renard P (2012) Mir-212/132 expression and functions: within and beyond the neuronal compartment. Nucleic Acids Res 40(11):4742–4753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Liang H (2015) Mir-132, Mir-15a and Mir-16 synergistically inhibit pituitary tumor cell proliferation, invasion and migration by targeting Sox5. Cancer Lett 356(2):568–578 [DOI] [PubMed] [Google Scholar]
- Wang W, Kwon E, Tsai L (2012) MicroRNAs in learning, memory, and neurological diseases. Learn Mem 19(9):359–368 [DOI] [PubMed] [Google Scholar]
- Wang J, Guan X, Guo F, Zhou J, Chang A, Sun B, Cai Y, Ma Z, Dai C, Li X, Wang B (2013) Mir-30e reciprocally regulates the differentiation of adipocytes and osteoblasts by directly targeting low-density lipoprotein receptor-related protein 6. Cell Death Dis 4:e845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Sundquist K, Hedelius A, Palmer K, Memon A, Sundquist J (2015) Circulating MicroRNA-144-5p Is associated with depressive disorders. Clin Epigenetics 7:69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber D, Gawrych K, Casjens S, Brik A, Lehnert M, Taeger D, Pesch B, Kollmeier J, Bauer T, Johnen G, Brning T (2017) Circulating Mir-132-3p as a candidate diagnostic biomarker for malignant mesothelioma. Dis Markers. 10.1155/2017/9280170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H, Yuan Y, Liu S, Wang C, Yang F, Lu Z, Wang C, Deng H, Zhao J, Shen Y, Zhang C, Yu X, Xu Q (2015) Detection of circulating miRNA levels in schizophrenia. Am J Psychiatry 172(11):1141–1147 [DOI] [PubMed] [Google Scholar]
- Willms E, Johansson H, Mager I, Lee Y, Blomberg K, Sadik M, Alaarg A, Smith C, Lehtio J, Andaloussi S, Wood M, Vader P (2016) Cells release subpopulations of exosomes with distinct molecular and biological properties. Sci Rep 6:22519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter J, Jung S, Keller S, Gregory R, Diederichs S (2009) Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol 11(3):228–234 [DOI] [PubMed] [Google Scholar]
- Wong J, Duncan C, Beveridge N, Webster M, Cairns M, Weickert C (2013) Expression of Npas3 in the Human cortex and evidence of its posttranscriptional regulation by Mir-17 during development, with implications for schizophrenia. Schizophr Bull 39(2):396–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Peng M, Yu M, Zhao Q, Li C, Jin Y, Jiang Y, Chen Z, Deng N, Sun H, Wu X (2015) Circulating microRNA Let-7d in attention-deficit/hyperactivity disorder. NeuroMol Med 17(2):137–146 [DOI] [PubMed] [Google Scholar]
- Wu S, Zhang R, Nie F, Wang X, Jiang C, Liu M, Valenzuela R, Liu W, Shi Y, Ma J (2016) MicroRNA-137 inhibits Efnb2 expression affected by a genetic variant and is expressed aberrantly in peripheral blood of schizophrenia patients. Ebiomedicine 12:133–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Zhao Y, Liu Y, Yang X, Yan M, Min Y, Pan Z, Qiu S, Xia S, Yu J, Yang P, Wan B, Shao Q (2018) Identifying MiRNA-MRNA regulation network of major depressive disorder in ovarian cancer patients. Oncol Lett 16(4):5375–5382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanez-Mo M, Siljander P, Andreu Z, Zavec A, Borras F, Buzas E, Buzas K, Casal E, Cappello F, Carvalho J, Colas E, Cordeiro-da Silva A, Fais S, Falcon-Perez J, Ghobrial I, Giebel B, Gimona M, Graner M, Gursel I, Gursel M, Heegaard N, Hendrix A, Kierulf P, Kokubun K, Kosanovic M, Kralj-Iglic V, Kramer-Albers E, Laitinen S, Lasser C, Lener T, Ligeti E, Line A, Lipps G, Llorente A, Lotvall J, Mancek-Keber M, Marcilla A, Mittelbrunn M, Nazarenko I, Nolte-t’ Hoen E, Nyman T, O’Driscoll L, Olivan M, Oliveira C, Pallinger E, del Portillo H, Reventos J, Rigau M, Rohde E, Sammar M, Sanchez-Madrid F, Santarem N, Schallmoser K, Ostenfeld M, Stoorvogel W, Stukelj R, Van der Grein S, Vasconcelos M, Wauben M, De Wever O (2015) Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles 4:27066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Hu Z, Du X, Davies H, Huo X, Fang M (2017) Mir-16 and fluoxetine both reverse autophagic and apoptotic change in chronic unpredictable mild stress model rats. Front Neurosci 11:428 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Young JJ, Silber T, Bruno D, Galatzer-Levy IR, Pomara N, Marmar CR (2016) Is there progress? An overview of selecting biomarker candidates for major depressive disorder. Front Psychiatry 7:72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Wu J, Zhang H, Zhang G, Sui J, Tong W, Zhang X, Nie L, Duan J, Zhang L, Lv L (2015) Alterations of Mir-132 Are novel diagnostic biomarkers in peripheral blood of schizophrenia patients. Prog Neuropsychopharmacol Biol Psychiatry 63:23–29 [DOI] [PubMed] [Google Scholar]
- Yuan H, Mischoulon D, Fava M, Otto M (2018) Circulating microRNAs as biomarkers for depression: many candidates, few finalists. J Affect Disord 233:68–78 [DOI] [PubMed] [Google Scholar]
- Zampetaki A, Mayr M (2012) Analytical challenges and technical limitations in assessing circulating miRNAs. Thromb Haemost 108(4):592–598 [DOI] [PubMed] [Google Scholar]
- Zeng L, He X, Wang Y, Tang Y, Zheng C, Cai H, Liu J, Fu Y, Yang G (2014) MicroRNA-210 overexpression induces angiogenesis and neurogenesis in the normal adult mouse brain. Gene Ther 21(1):37–43 [DOI] [PubMed] [Google Scholar]
- Zhang J, Li S, Li L, Li M, Guo C, Yao J, Mi S (2015) Exosome and exosomal microRNA: trafficking, sorting, and function. Genom Proteom Bioinform 13(1):17–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhao Y, Tian C, Wang J, Li W, Zhong C (2018) Differential exosomal microRNA profile in the serum of a patient with depression. Eur J Psychiatry 32(3):105–112 [Google Scholar]
- Zhao C, Sun G, Ye P, Li S, Shi Y (2013) MicroRNA Let-7d regulates the Tlx/MicroRNA-9 cascade to control neural cell fate and neurogenesis. Sci Rep 3:1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucchi FC, Yao Y, Ward ID, Ilnytskyy Y, Olson DM, Benzies K, Kovalchuk I, Kovalchuk O, Metz GA (2013) MateRNAl stress induces epigenetic signatures of psychiatric and neurological diseases in the offspring. PLoS ONE 8(2):e56967 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.