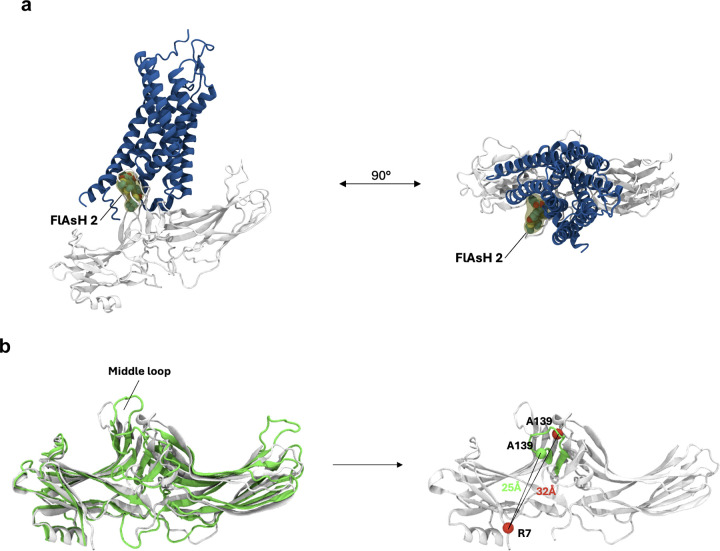
Extended Data Figure 4: Orientation of FlAsH 2 in different configurations of receptor-β-arrestin1 complex.
(a) Spatial orientation of FlAsH 2 in a β-arrestin 1-GPCR core complex. Using the structure of the neurotensin receptor 1 (blue) in complex with β-arrestin 1 (gray) (PDB: 6UP7), we manually modelled the full-length FlAsH 2 into the complex (yellow). Subsequently, the structure was subjected to a short minimization run (0.1 RMS kcal/mol/Å2 gradient, Amber10:EHT forcefield). The resulting structural model suggests that in the GPCR core complex, FlAsH 2 would intercalate into the receptor structure, thus reducing its mobility and resulting in a low BRET ratio. (b) Spatial orientation of FlAsH 2 in a β-arrestin 1-GPCR C-tail complex. Comparison of the structures of the V2Rpp-bound (green, PDB: 4JQI) and inactive (white, PDB: 1G4M) β-arrestin 1. The position of the insertion of FlAsH 2 (A139) within the middle loop is depicted as a sphere (green – 4JQI, red – 1G4M). The distance between FlAsH 2 and the NLuc donor was approximated by plotting the distance between the insertion position and the N-terminally located R7.