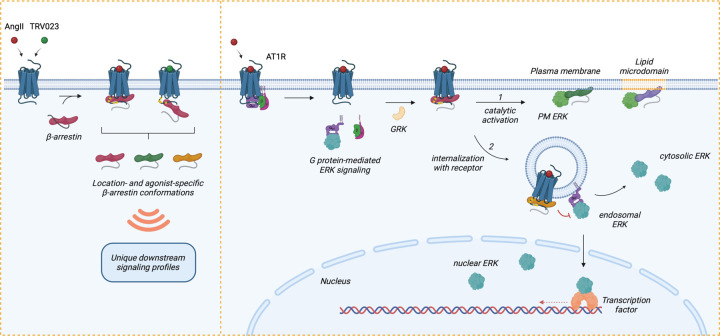
Figure 6: Model of regulation of location-biased ERK activation downstream of AT1R.
Upon stimulation with agonists, AT1R activates Gαq and Gαi, resulting in the dissociation of the G protein subunits and promoting endosomal ERK signaling (after receptor internalization mediated by β-arrestins), which can propagate into the cytosol and nucleus. Following G protein activation, GRKs phosphorylate the receptor C-tail, which promotes the recruitment of β-arrestins. Depending on the agonist, β-arrestins can form two main complexes with the receptor: a core conformation induced by AngII and a hanging tail conformation induced by TRV023. Each configuration stabilizes distinct conformational profiles of receptor-bound β-arrestins. Subsequently, β-arrestins either (1) undergo catalytic activation by the receptor, allowing them to translocate across the PM and lipid microdomains independently of the receptor, or (2) co-traffic with the internalized receptor into endosomes. Depending on the membrane lipid environment and subcellular locations, catalytically active β-arrestins and receptor-associated β-arrestins adopt unique conformational signatures that dictate their function, such as regulating the ERK/MAPK signaling pathway. While ERK activity at the PM is mainly promoted by catalytically active β-arrestins, endosomal and nuclear ERK signaling is activated by Gαq and Gαi and can be inhibited by β-arrestins. Receptor endocytosis distributes subcellular pools of ERK signaling from the PM into the endosome, cytosol, and nucleus. In summary, β-arrestin plays critical roles in regulating the intensity, duration, and location bias of AT1R signaling through desensitization, internalization, and catalytic activation initiating its own pattern of ERK signaling, thus modulating the cellular response.