Fig. 4 |. Classification of endogenous biomarkers of hepatic and renal transporters and International Transporter Consortium recommendations for their application in drug development.
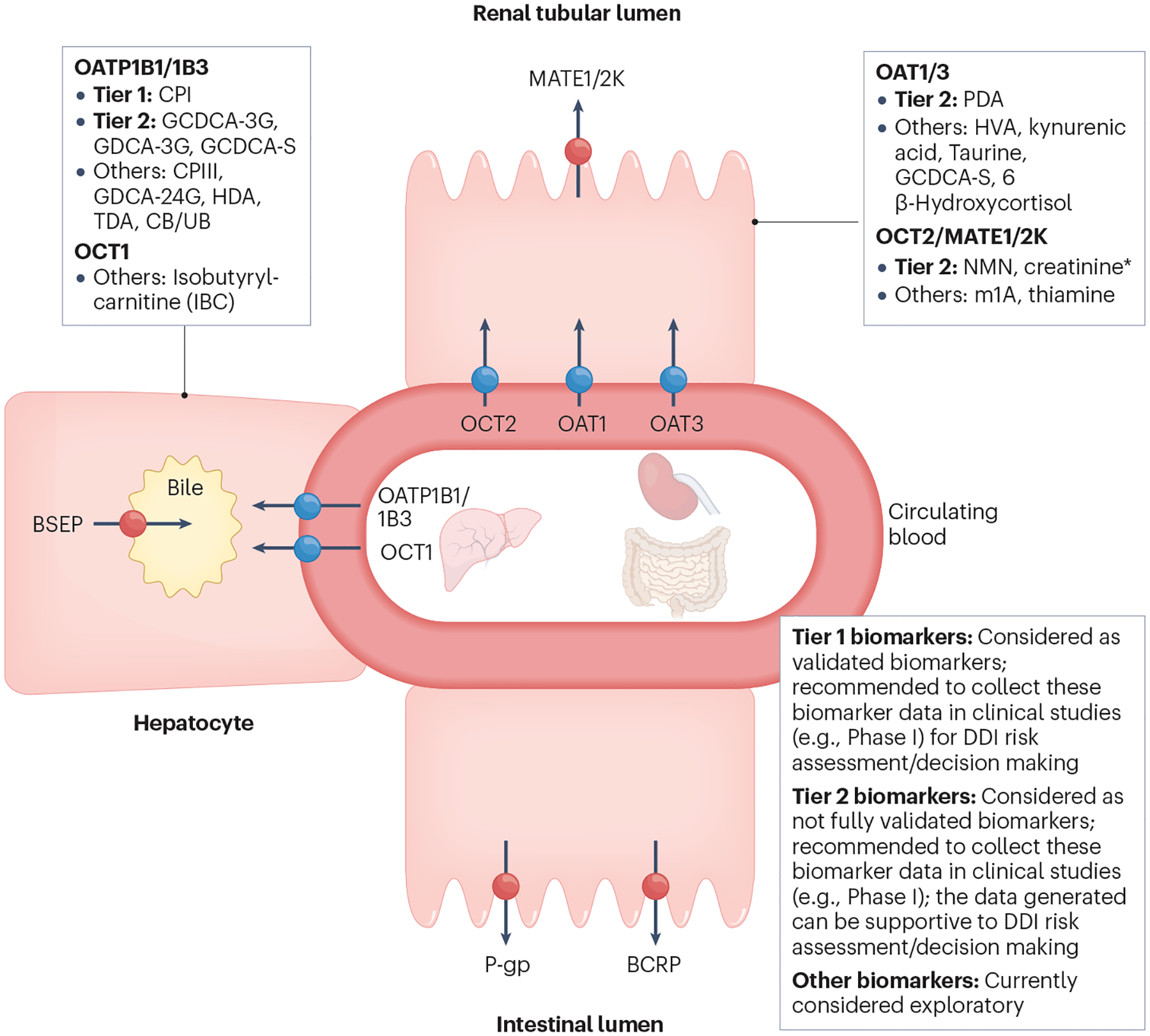
Tier 1 biomarkers: Recommendation to include these biomarkers in clinical Phase I studies when in vitro studies show clinical drug–drug interaction (DDI) potential; considered validated for clinical DDI risk assessment (see Fig. 5 for decision tree). Biomarkers have (1) high sensitivity/selectivity to the transporter of interest (based on in vitro phenotyping or clinical pharmacogenomic data); (2) available clinical DDIs with potent, moderate, weak and non-inhibitors; (3) validated DDI prediction performance with probe drugs; and (4) available mechanistic models. Tier 2 biomarkers: Recommendation to collect data on these biomarkers in clinical Phase I studies when in vitro studies show clinical DDI potential; not considered validated for clinical DDI risk assessment/decision making yet. Biomarkers have (1) high sensitivity/selectivity to the transporter of interest (based on in vitro phenotyping and clinical pharmacogenomic data); (2) limited available clinical DDIs with potent, moderate, weak and non-inhibitors; and (3) models developed for some, but not all Tier 2 biomarkers. Further evaluation is required to understand their DDI prediction performance. * NMN may serve as a more selective and sensitive biomarker than creatinine owing to a higher contribution of active renal secretion clearance to the total clearance (~70% vs. ~30%). Creatinine is included in Tier 2 because of the availability of data and its routine measurement to monitor renal toxicity. Elevation of serum creatinine may also be caused by reduced renal function, and it is important to distinguish the inhibition of OCT2/MATE versus renal toxicity. Other biomarkers: Currently not recommended to collect data on these biomarkers in clinical Phase I studies due to relatively low sensitivity/selectivity or limited data to understand biomarker selectivity/sensitivity or limited clinical reports evaluating DDI predictive performance. BCRP, breast cancer resistance protein; BSEP, bile salt export pump; CB, conjugated bilirubin; CPI, coproporphyrin I; CPIII, coproporphyrin III; GCDCA-3G, glycochenodeoxycholic acid-3-glucuronide; GCDCA-3S, glycochenodeoxycholic acid-3-sulfate; GDCA-3G, glycodeoxycholic acid-3-glucuronide; GDCA-24G, glycodeoxycholic acid-24-glucuronide; HDA, hexadecanedioate; HVA, homovanillic acid; MATE, multidrug and toxin extrusion protein; m1A, N1-methyladenosine; NMN, N1-methylnicotinamide; OAT, organic anion transporter; OATP, organic anion transporting polypeptide; OCT, organic cation transporter; PDA, 4-pyridoxic acid; P-gp, P-glycoprotein; TDA, tetradecanedioate; UB, unconjugated bilirubin. Adapted with permission from ref. 4, Wiley.
