Fig. 5 |. Decision tree for organic anion transporting polypeptide (OATP1B)- mediated drug–drug interaction risk assessment with coproporphyrin I.
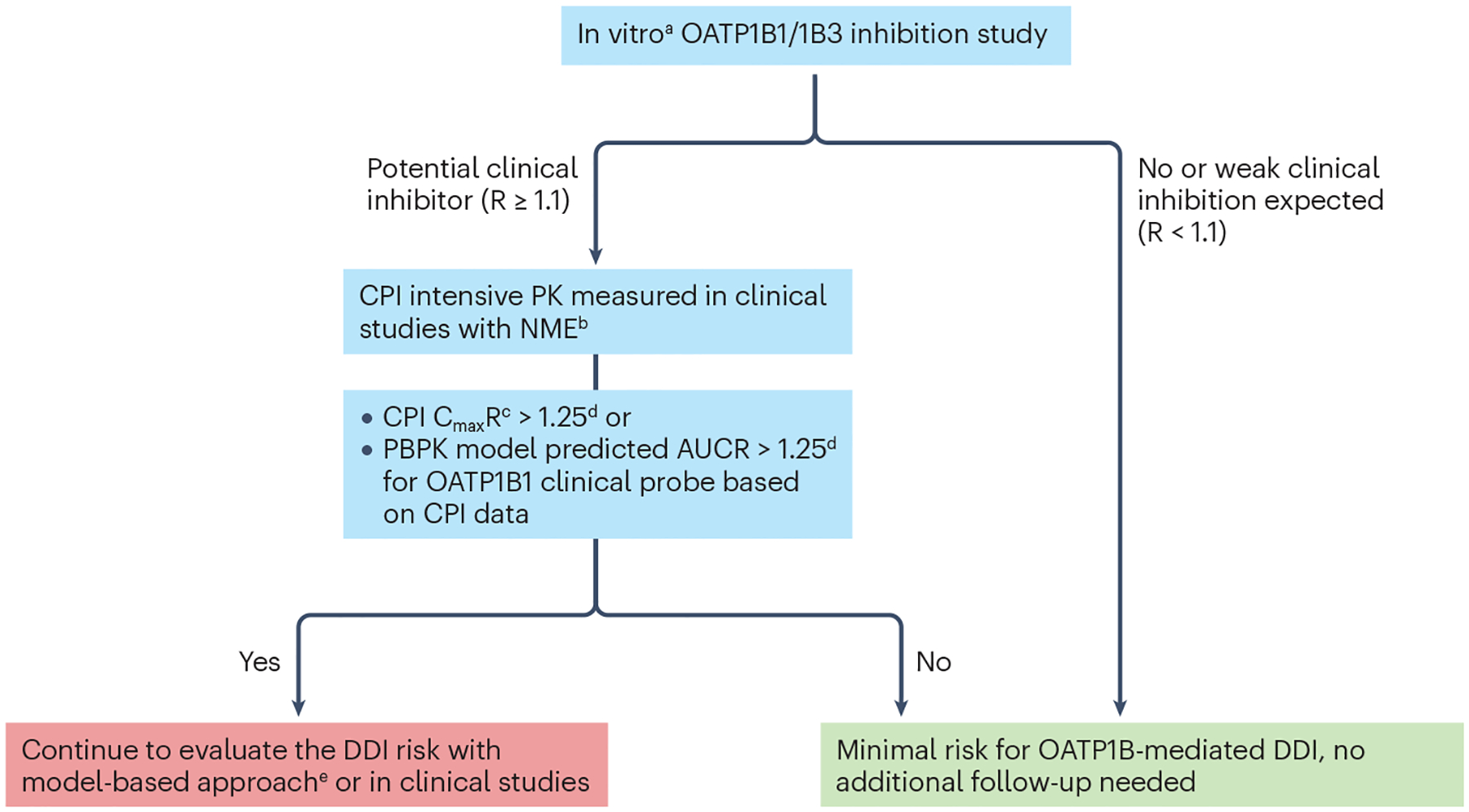
aConsidering substrate-dependent inhibition often seen in the case of OATP1B1, it is recommended to generate in vitro inhibition data with coproporphyrin I (CPI) as a Tier 1 biomarker and a relevant co-medication of interest for a new molecular entity (NME). R is the predicted ratio of the victim drug’s area under the plasma concentration-time profile (AUC) in the presence and absence of the investigational drug as OATP1B inhibitor. bTime-matched biomarker concentrations in the absence of the NME are usually not available from firstin- human or clinical pharmacology studies except for drug–drug interaction (DDI) studies. There are two potential approaches to address this issue, namely, one is to use the pre-dose single time point as the baseline level, and the other is to use data from a separate placebo cohort. The first approach is useful for CPI as there is little to no diurnal variation; however, one must be careful when comparing biomarker kinetics at the steady-state of the NME compared with the pre-dose biomarker data. The second approach is valid except that there is less power to detect an interaction with the parallel, non-crossover comparison. cThe appropriate metrics depends on the kinetic properties of both biomarkers and the NMEs4. Because CPI has a short terminal half-life, AUC is less appropriate, as the ratio of AUC depends on the duration for which AUC is calculated, and this can lead to under-estimation of the magnitude of inhibition compared with CmaxR, as seen in CPI kinetics in the presence of GDC-0810 or cyclosporine A184,232. dOther thresholds can be justified based on the exposure-response relationships of the co-medications of interest for the NME. eFactors that increase confidence in quantitative DDI prediction with model-based approaches: (1) CPI data from dose-ranging trials, especially those including supratherapeutic dose, (2) CPI observations from a sufficiently large number (for example, >10 participants receiving the same dose of the NME as typically seen in a dedicated DDI study) and (3) consistent observations with other biomarkers such as GCDCA-3G. AUCR, AUC of CPI in the presence of an inhibitor relative to the baseline AUC (control); CmaxR, ratio of CPI Cmax in the presence of an inhibitor relative to the baseline Cmax (control); PK, pharmacokinetics.
