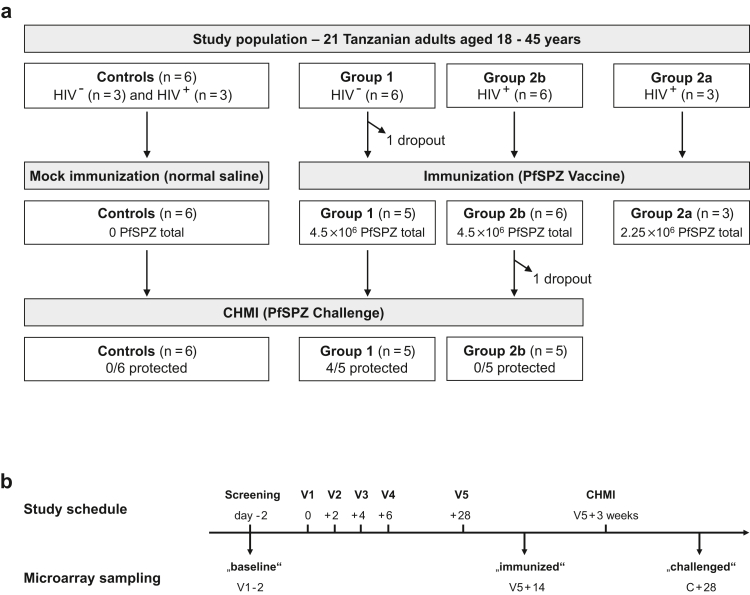
Fig. 1.
Study design and sampling time points for microarray analysis. (a) Volunteer group allocation. The trial included an HIV positive pilot group (group 2a) that received 4.5 × 105 PfSPZ of PfSPZ Vaccine in each inoculation and did not undergo CHMI. Group 1 and group 2b comprised each six HIV negative and HIV positive volunteers that received five times 9 × 105 of PfSPZ Vaccine in direct venous inoculation. The placebo control group had six volunteers that were HIV negative (n = 3) or HIV positive (n = 3). (b) Serum sample collection and study flow chart. Serum samples for microarray analysis were collected at baseline (V1–2), 14 days after the fifth injection (V5 + 14) and 28 days after CHMI (C + 28).