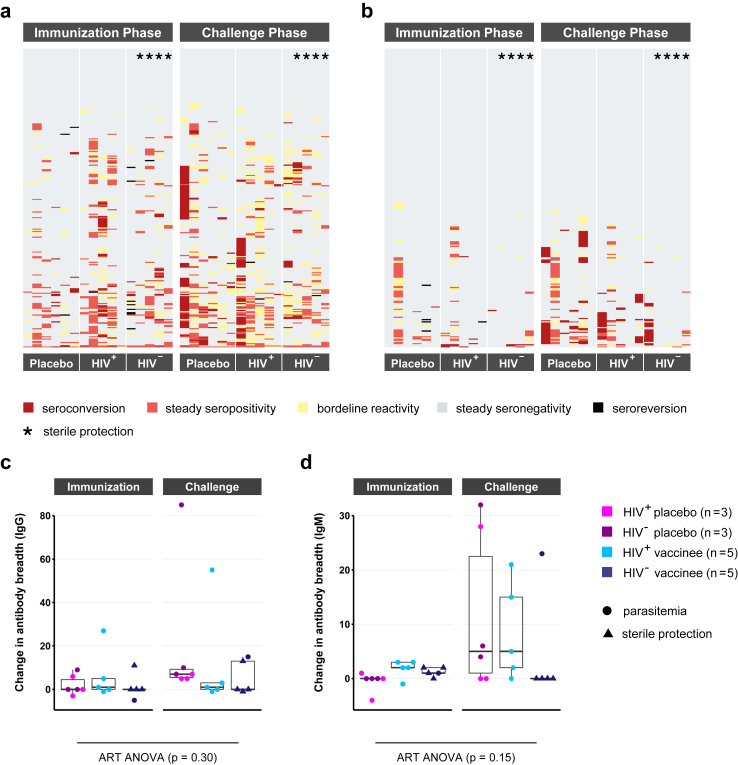
Fig. 7.
Antibody acquisition and change in antibody breadth. Changes in antibody repertoire over the immunisation and challenge phase were compared between placebo recipients (n = 6), HIV positive (n = 5) and HIV negative vaccinees (n = 5). The individual antibody breadth gives the number of seropositive antigens at a certain time point (signal intensity of >3 log2-levels above a malaria-naïve control). Seroconversion, the acquisition of a novel antigen, was defined as exceeding the seropositivity threshold accompanied by a signal increase of >2 log2-levels between the time points compared. Seroreversion was defined as drop below the threshold with a signal decrease of >2 log2-levels. Smaller signal fluctuations around the threshold were designated as borderline reactivity. (a, b) Changes in antigen recognition after immunisation and challenge are shown as heatmap for IgG (a) and IgM (b) with antigens depicted in rows and samples in columns. (c, d) Changes in the IgG (c) and IgM (d) antibody breadth over immunisation and challenge are compared between the three study groups of placebos, HIV positive and negative vaccinees. The influence of study group and phase on the change in antibody breadth was evaluated using an Aligned Rank Transform (ART) ANOVA model. The boxplots give median antibody breadths, interquartile ranges (IQR) and whiskers of length 1.5 × IQR.