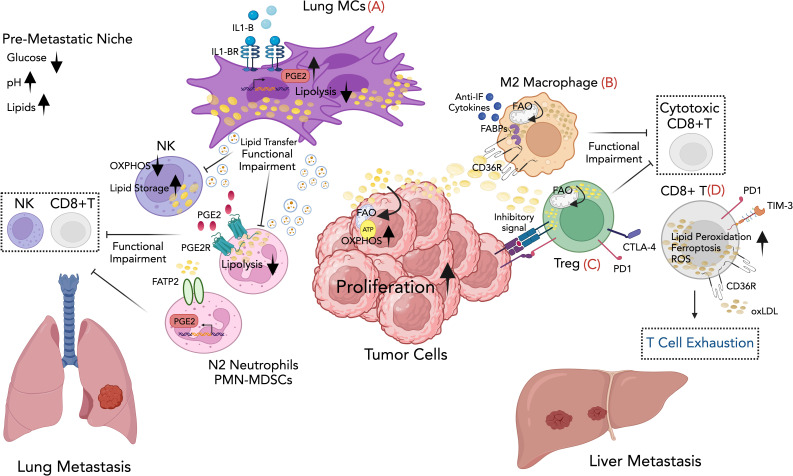
Figure 1.
Graphical overview for some of the mechanisms involving lipid immunomodulation of metastatic growth described in the review. (A) Lung mesenchymal cells in the lung facilitate breast cancer metastasis by suppressing anti-tumor immunity and providing metabolic support to the metastatic tumor cells through lipid transfer to both tumor cells and NK cells via exosome-like vesicles, leading to enhanced survival and proliferation of tumor cells and dysfunction of NK cells. Lipids stored in lung neutrophils are transported to metastatic tumor cells through a macropinocytosis-lysosome pathway, endowing tumor cells with augmented survival and proliferative capacities. (B) Lipid-rich vesicles are selectively taken up by macrophages through CD36, providing them with fuel and activating their tumor-promoting functions. The accumulation of lipid droplets within the macrophages supports the M2 phenotype, which is characterized by the production of anti-inflammatory cytokines and the suppression of anti-tumor immune responses. (C) Tregs adapt metabolically to harsh TME, using fatty acids for fueling fatty acid oxidation (FAO). This adaptation enables Tregs to promote tumor progression and metastasis through immune evasion. (D) Fatty acid uptake by CD8+ T TILs causes lipid peroxidation and ferroptosis, weakening their anti-tumor activity and supporting cancer metastasis. Created with BioRender.com.