Introduction
Efforts to describe and classify disorders of consciousness date back more than 2 millennia. In 400 B.C., Aristotle offered a simple taxonomy that was centered on self-awareness and distinguished sleep from unconsciousness–“What is (inanimate) is unaware, while what is (animate) is not unaware … While asleep, the critical activities, which include thinking, sensing, recalling and remembering, do not function as they do during wakefulness.”1,2 While the construct of consciousness is conceptually grounded in the dichotomy between self-awareness and unawareness, Plum and Posner3 eloquently posed the central problem, “we can only infer the self-awareness of others by their appearance and their acts.” This observation also highlights a critically important limitation of the current diagnostic scheme-all rely almost exclusively on behavioral evidence. Overreliance on behavioral observations and inferential judgement has resulted in confusion and misunderstanding, contributing to alarmingly high rates of diagnostic error.4–6
How should we conceptualize a DoC taxonomy that would inform clinical practice and advance the field? Such a taxonomy should aspire to accomplish four aims.7 Chief among these, it should promote communication between clinicians, and among clinicians, family members, and surrogate decision-makers. This requires the uniform use of diagnostic terms and clinical criteria. Recent multi-organizational guidelines in the U.S., Europe, and U.K. have moved closer to this goal but it has not yet been achieved. Second, a clinically useful taxonomy should demonstrate “goodness of fit.” That is, the language used should accurately describe and clearly distinguish the conditions of interest. At present, the existing literature is replete with different terms that refer to the same condition and there is overlap in clinical features that purport to describe distinct conditions. Third, the taxonomy should be feasible to implement. All settings that provide care for this population should have access to the personnel, procedures, and tools that are necessary to identify and classify DoC. This is a second major limitation of present day DoC care. A notable example is the recently defined cognitive motor dissociation (CMD) syndrome, which requires the use of specialized functional neuroimaging acquisition and analytic procedures that are generally only available in academic medical centers.8 The fourth attribute of an effective taxonomy for DoC is that it should facilitate clinical management decisions. The prevailing approach to the treatment of patients with DoC is trial and error. Unique behavioral features and the underlying pathophysiologic substrate are rarely considered in treatment selection, resulting in inconsistency and imprecision in the approach. A rational taxonomy should incorporate knowledge of the neurobiological mechanism underlying the behavioral phenotype as this would inform optimal treatment targets and the likelihood of therapeutic response.
We view the four aims discussed above as aspirational but believe there is a pressing need to move toward a more rational approach to DoC taxonomy. In this article, we provide a brief historical review the major DoCs and then discuss the prevailing operational definitions and diagnostic criteria for each. We rely heavily on current practice guidelines, identify knowledge gaps and, where appropriate, discuss future directions that may have a favorable impact.
Neurobiological Considerations
Existing international practice guidelines recognize four major DoCs: coma, vegetative state (VS) (also referred to as unresponsive wakefulness syndrome (UWS)), minimally conscious state (MCS), and post-traumatic confusional state (PTCS) (also referred to as emergence from MCS (eMCS)).9–11 Disturbance in consciousness is typically caused by either focal injury to the brain stem or diffuse bi-hemispheric injury that results in the widespread disruption of cortical networks responsible for the regulation of arousal and internal and external awareness.12,13 DoCs may occur following direct insult to specific structures that are integral to sustaining arousal and awareness or indirectly through loss of connectivity between key structures. While the fundamental neurobiological mechanisms required for conscious awareness remain poorly understood, recovery is closely associated with the location and volume of structural damage to the brain.14
A major scientific and clinical problem associated with the assessment of DoC is the phenomenon of fluctuation in “state.” That is, the level of consciousness may vary moment to moment in patients with DoC. For example, it is not uncommon for a clinician to observe clear and convincing evidence of conscious awareness through command-following on the examination conducted in the morning and then fail to elicit the same behavior on reassessment later in the same day. The pathophysiologic mechanism underlying these fluctuations is unclear but has been linked to instability in the arousal regulation and under-activation of downstream cortical fields brought about by the deafferentation of the central lateral thalamus.15,16 This instability in the arousal regulation system complicates DoC classification from a diagnostic standpoint as patients may transition in and out of different disorders from examination to examination. While the 40% diagnostic error rate repeatedly reported in DoC literature4–6 partially reflects insufficient knowledge and misunderstanding among clinicians, it is likely that pathophysiologic oscillations in thalamic gating systems also play a key role.
Challenges to Establishing a Uniform Taxonomy
Over the last three decades, multiple professional organizations10,17–20 have proposed recommendations for defining and diagnosing DoCs. Earlier efforts were conducted in parallel, sometimes leading to conflicting guidance.21 More recent initiatives have been decidedly more interdisciplinary, leading to greater consistency across recommendations. There has also been a discernible shift from consensus-based to evidence-based practice recommendations.9,10 One notable example is the evidence-based recommendations for practice co-sponsored by the American Academy of Neurology (AAN), American Congress of Rehabilitation Medicine (ACRM), and the National Institute on Disability, Independence, and Rehabilitation Research and published concurrently in Neurology9 and Archives of Physical Medicine and Rehabilitation in 2018.22 This partnership between neurology and neurorehabilitation organizations was successful in achieving consensus to discontinue use of the term, permanent vegetative state, which had previously been an enduring source of controversy between the 2 disciplines.
Despite increased interdisciplinary collaboration, there is ongoing disagreement on the terminology and diagnostic criteria associated with these conditions, posing considerable clinical and ethical risks to clinicians, patients, and caregivers concerned and living with DoC. Lack of standardization in terminology fosters inappropriate management, inaccurate prognostication, increased caregiver stress, and societal nihilism.23 Even when there is general consensus among different professional disciplines, widescale adoption and implementation of new recommendations is often slow or incomplete due to delays in knowledge translation, poor dissemination across medical, scientific, and social platforms, and subtle differences in nomenclature.
The remainder of this article provides an historical review of the terminology, definitions, and diagnostic criteria associated with coma, VS, MCS, and PTCS. Fig. 1 provides a timeline of key developments that have influenced DoC classification. Fig. 2 shows the distinguishing features of the DoCs covered in this article. Throughout the article, we highlight important knowledge gaps and future directions that may move the field toward a more rational taxonomy for DoC.
Fig. 1.
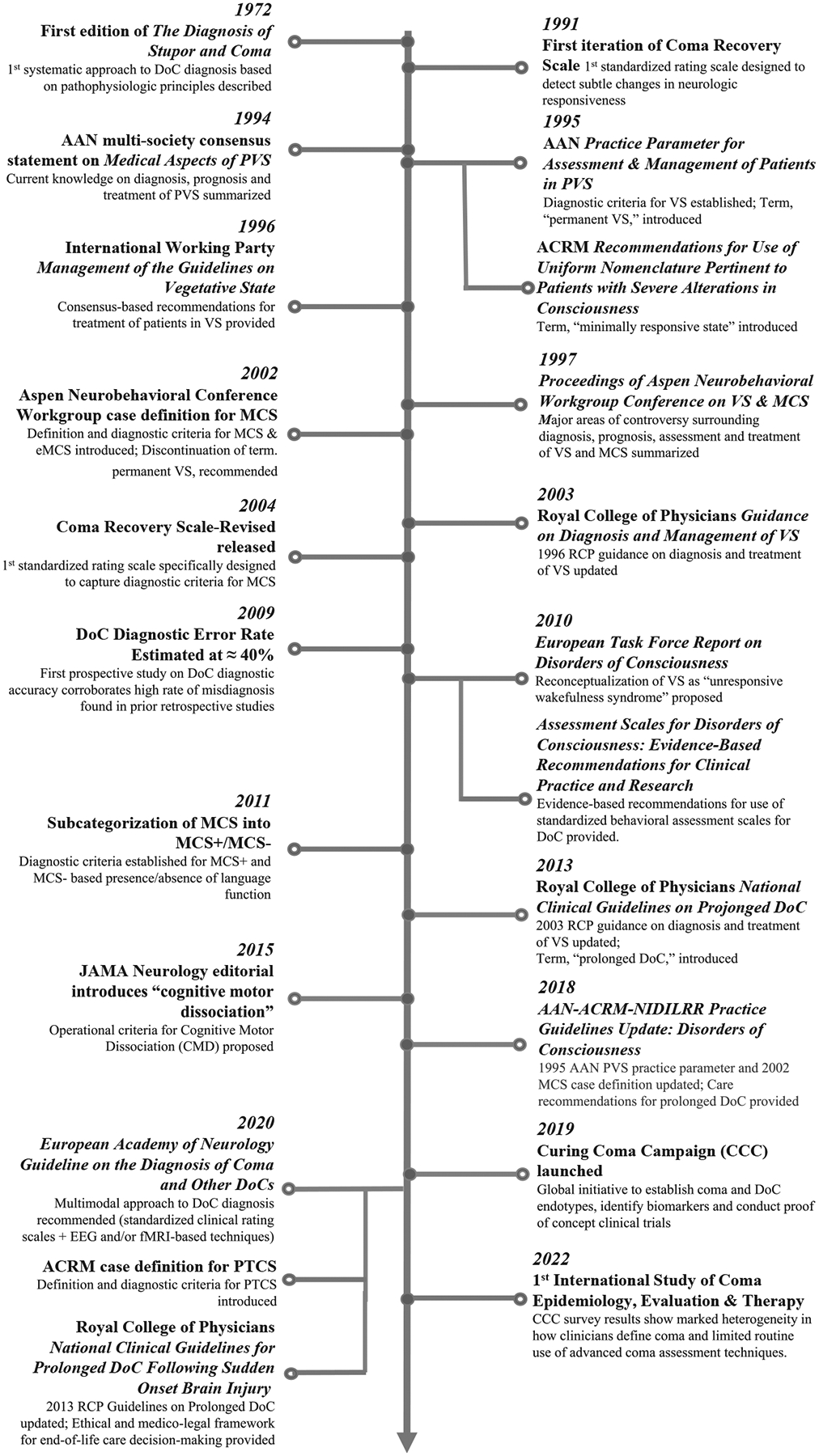
Timeline of events influencing DoC taxonomy over the past five decades. AAN, American Academy of Neurology; ACRM, american congress of rehabilitation medicine; CCC, curing coma campaign; DoC, disorder(s) of consciousness; EEG, electroencephalography; eMCS, emerged from minimally conscious state; fMRI, functional magnetic resonance imaging; MCS−, minimally conscious state-minus; MCS, minimally conscious state; MCS+, minimally conscious state-plus; PTCS, post-traumatic confusional state; PVS, persistent vegetative state; RCP, royal college of physicians; VS, vegetative state.
Fig. 2.
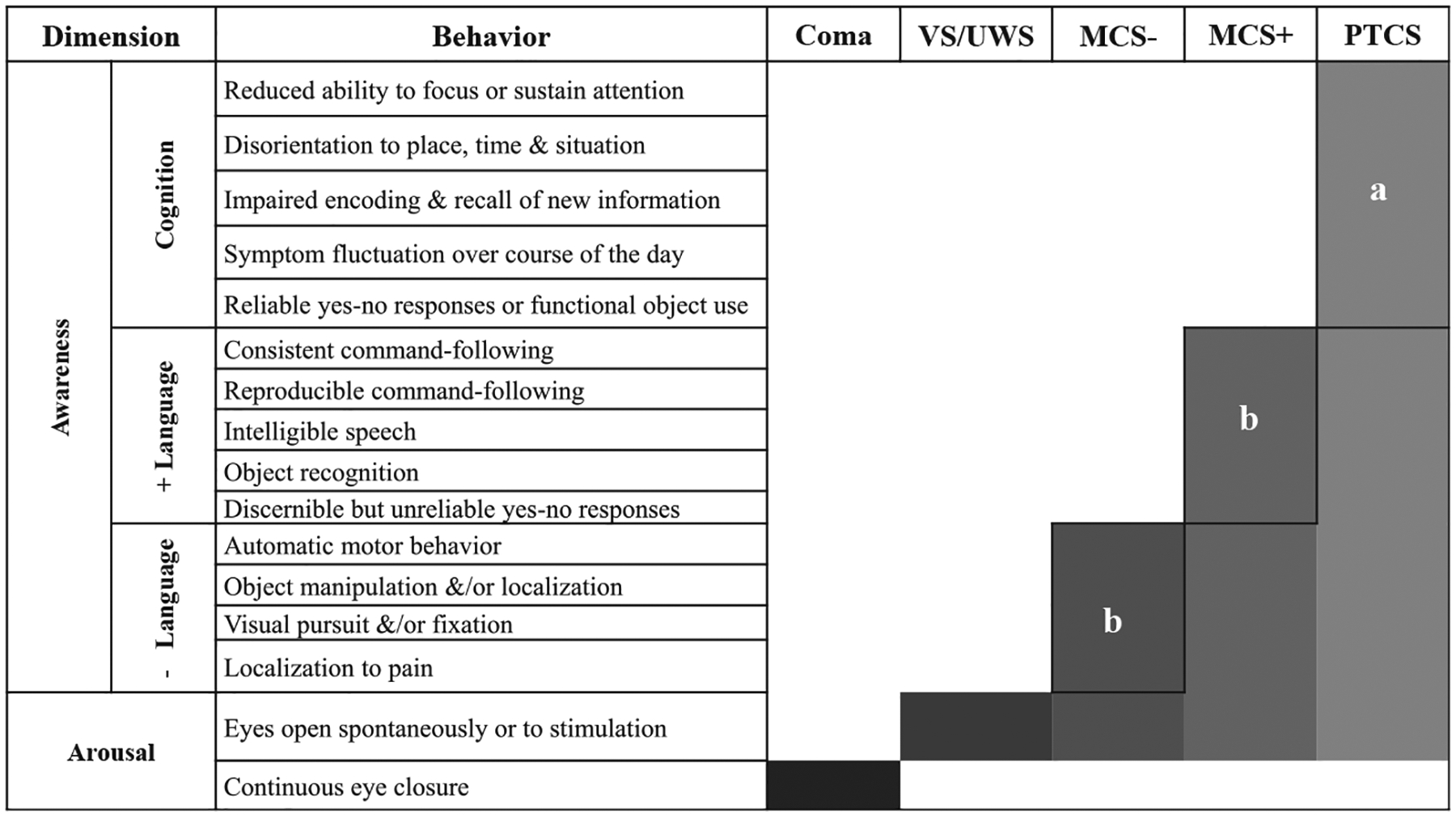
Differential diagnostic criteria for disorders of consciousness. Behaviors associated with DoCs can be conceptualized along two dimensions-arousal (or wakefulness) and awareness (ie, perception of self and environment). The shaded regions of the bars indicate the distinguishing characteristics of each disorder. MCS−, minimally conscious state; MCS+, minimally conscious state plus; PTCS, post traumatic confusional state; VS, vegetative state. aAll behaviors are required. bAt least one of these behaviors must be present. (Adapted from Giacino JT, Katz D, Schiff N, Bodien YG. Assessment and Rehabilitative Management of Individuals with Disorders of Consciousness. In: Zasler ND, Katz DI, Zafonte RD, editors. Brain Injury Medicine: Principles and Practice- 3rd edition. New York: Springer Publishing Company, 2022. p. 447–461, with permission.)
Coma
The condition widely referred to as, “coma,” is of Greek origin and derives from “koma,” meaning deep sleep. Many other terms have been suggested to describe this condition, anchoring to either the behavioral or neuropathologic features.24 For example, the term, cataphora, refers to a hyper-somnolent state while, apallic syndrome, introduced by Kretschmer in 1940, connotes, “without cortex.”25
In 1972, Plum & Posner published their seminal work, The Diagnosis of Stupor and Coma, offering the first detailed description of the pathophysiologic and clinical characteristics of coma.3 In subsequent editions, the diagnostic features of coma have largely remained the same. Coma is described as a state of pathological unconsciousness, characterized by complete loss of spontaneous arousal, sustained eye closure despite the introduction of noxious stimulation, and total absence of behaviors associated with “normal” consciousness.3,13 Neuropathologic studies completed by Plum, Posner, and others have demonstrated that coma can result from severe, diffuse, bi-hemispheric damage to the cortex, underlying white matter, thalamus, or focal lesions of the paramedian tegmentum.13,26,27 Persons in coma typically remain in this state for approximately 2 to four weeks before transitioning to higher levels of consciousness.
Recently, expert consensus-based criteria for diagnosing coma were established under the auspices of the Curing Coma Campaign (CCC). The CCC is comprised of a multidisciplinary international panel of experts, including scientists, neurointensivists, neurorehabilitation specialists, and implementation scientists, who have established a “coma community” with the primary aim of advancing coma science.28 The expert panel identified the following cardinal features of coma (Box 1).
Box 1. Diagnostic criteria for coma.
All of the following criteria must be met to establish the diagnosis of coma:
No command-following;
No intelligible speech or recognizable gesture;
No volitional movement (reflexive movement such as extensor or flexor posturing, withdrawal from pain, triple flexion may occur);
No visual pursuit, fixation, saccade to stimuli, or eye opening or closing to command;
The above criteria are not due to the use of the paralytic agent, active use of sedatives, another neurologic or psychiatric disorder (eg, locked-in syndrome, neuromuscular disorder, catatonia, akinetic mute, abulia, conversion disorder); and
The patient does not have evidence of cognitive motor dissociation (ie, the covert ability to follow commands) based on electrophysiological or functional imaging, if such testing is available.29
The clinical criteria recommended by both Plum and Posner and the CCC expert panel have an important limitation–they center on the absence of specific behaviors. The recently defined condition referred to as cognitive motor dissociation (CMD), in which consciousness is retained in the absence of behavioral evidence (see CMD discussion later in discussion) attests to the inadequacy of these criteria.8 Moreover, Kondziella and Frontera have argued that some comatose patients may retain spontaneous or stimulus-induced eye-opening, defying traditional diagnostic criteria, which emphasize eye closure as a primary feature, and challenge the longstanding notion that coma is incompatible with arousal.30 The authors suggest a putative pathophysiologic mechanism for eyes-open coma and call for autopsy and functional imaging studies to further investigate this presentation as a new coma phenotype.
Despite significant advancements in neuroimaging technology, the necessary and sufficient lesion profile underlying coma remains elusive and there is no consensus on the defining clinical characteristics.27 In 2022, CCC collaborators published the results of an international crowdsourcing survey, the first of its kind, to investigate the point prevalence of coma in the United States and United Kingdom. Findings revealed that the point prevalence rate was approximately four times higher in the U.S. than in the U.K. (31 cases per 100,000 in the U.S. v. 7 cases per 100,000 in the U.K.).31 Also in 2022, the CCC published the results of the Come Together Survey, which was designed to assess global perspectives on the definition, clinical features, and management strategies associated with coma.29 Survey results indicated that only 64% of respondents agreed with the cardinal features of coma recommended by the expert panel. There was also substantial variability in assessment methods and treatment strategies routinely used. Findings from this study highlight the pressing need to revisit prevailing diagnostic criteria and management strategies for coma. Current and future efforts to improve longitudinal follow-up with patients who experience coma is also needed to adequately inform policies, protocols, and minimal competency guidelines for equitable management of coma. Furthermore, the CCC is partnering with the National Institute of Health to develop unique common data elements (CDE), or a shared language, for coma.32 CDEs for coma will streamline standardization and foster international consensus on operational definitions and diagnostic criteria for coma.33
Vegetative State
The roots of VS can be tracked back to Aristotle’s introduction of the “vegetative faculty” in De Anima (On the Soul)2 from the mid-4th century B.C.E.34 This early conceptualization sparked a French physician, Xavier Bichat (1771–1802),35 and an American neurologist/endocrinologist, Walter Timme (1874–1956),36 to extend the concept of a vegetative nervous system. The origin of the concept of vegetative functions emphasized control of autonomic, internal organ systems, such as digestive, cardiovascular, and respiratory processes, which are mediated by the brainstem.34,37 These functions were classified as foundational, simplistic, automatic internal processes, dichotomized from external processes, which were thought to be responsible for voluntary expression and relation to one’s environment.
In 1963, Arnaud and colleagues38 referred to patients in the early stages of recovery from severe brain injury as, “vie vegetative,” denoting “vegetative life.” In 1971, in discussing prognostic indicators following severe brain injury, Vapalhati and Troupp modified usage of this term to suggest “vegetative survival.”39 In 1972, Bryan Jennett, a neurosurgeon from Glasgow, and Fred Plum, an American neurologist, published their seminal article entitled, “Persistent Vegetative State After Brain Damage” in Lancet, introducing persistent vegetative state (PVS) as a diagnostic term.40 The term, “persistent,” was added as a modifier for VS to distinguish patients who were observed to experience prolonged wakefulness without awareness from patients who quickly transitioned to higher states of consciousness following initial injury.40 Jennett and Plum presciently recognized the potential negative implications of adding “persistent” to VS stating: “Certainly we are concerned to identify an irrecoverable state, although the criteria needed to establish that prediction reliably have still to be confirmed. Until then “persistent” is safer than “permanent” or “irreversible”; but prolonged is not strong enough, and unless it is quantified it is meaningless (p. 735).”40 In the succeeding years, the definition of PVS was changed to denote an “irreversible state,” resulting in clinical confusion and inconsistency in the literature.
In 1994, the American Academy of Neurology (AAN) organized the Multi-Society Task Force on the Vegetative State and published the landmark article, “Medical Aspects of the Persistent Vegetative State,” as a 2-part article in the New England Journal of Medicine.17,18 The Task Force report clarified that persistent VS was applicable when this condition persisted for at least 1-month post-injury. The authors also introduced the prognostic term, permanent vegetative state, to describe patients with an “exceedingly small” chance of regaining consciousness.17,18,41 It was recommended that this term be applied after 12 months following traumatic brain injury (TBI), and after three months following non-traumatic injury. One year after the publication of the AAN Task Force Report, the American Congress of Rehabilitation Medicine (ACRM) published, “Recommendations for Use of Uniform Nomenclature Pertinent to Patients with Severe Alterations in Consciousness.” This position statement advocated for the discontinuation of the term, persistent VS, and recommended instead that the diagnosis of VS be accompanied by the length of time post-injury as this would convey prognostic information.19 Around the same time, the UK-lead “International Working Party on the Management of the Vegetative State: Summary Report” was published by an international group of neurologists, neurosurgeons, neuropsychologists, and neurorehabilitation specialists. The International Working Party proposed three vegetative presentations: hyporesponsive state, reflexic responsive state, and localizing responsive state. The International Working Party recommendations differed from the Multi-Society Task Force guidelines in that the former included localizing responses, including visual tracking, as a feature of the third VS category.20 Although each group shared the intention of clarity and harmonization of nomenclature, these initiatives were conducted in parallel, resulting in significant differences in nomenclature and defining features. In 2002, the Aspen Neurobehavioral Workgroup published a consensus statement resolving prior discrepancies in some recommendations, including the definition of persistent VS.42–44 Recent longitudinal studies now clearly indicate that recovery from VS can occur years after injury, substantiating discontinuation of the term, permanent VS.45
Diagnostic Criteria of the Vegetative State
Vegetative state (VS) is characterized by intermittent periods of wakefulness, evidenced by either spontaneous or stimulus-induced arousal, despite the absence of behavioral signs of conscious awareness (Box 2).17,18 Return of eye opening in patients in VS is the indicative of restoration of reticular system function. Additionally, patients in VS typically do not require mechanical ventilation as the recovery of the reticular system preserves life-sustaining, autonomic functions. Sleep-wake cycles are typically present on EEG.
Box 2. Diagnostic criteria of the vegetative state.
All of the following criteria must be met to establish the diagnosis of VS:
No evidence of awareness of self or environment and an inability to interact with others;
No evidence of sustained, reproducible, purposeful, or voluntary behavioral responses to visual, auditory, tactile, or noxious stimuli;
No evidence of language comprehension or expression;
Intermittent wakefulness manifested by the presence of sleep-wake cycles;
Sufficiently preserved hypothalamic and brain-stem autonomic functions to permit survival with medical and nursing care;
Bowel and bladder incontinence; and
Variably preserved cranial-nerve reflexes (pupillary, oculocephalic, corneal, vestibulo-ocular, gag and spinal reflexes.17
Is Vegetative State a State or Syndrome ?
There is ongoing disagreement as to whether VS should be characterized as a “state” or a “syndrome.” The Oxford English Dictionary defines a state as, “the particular condition that someone or something is in at a specific time”46 whereas a syndrome represents “a group of symptoms which consistently occur together, or a condition characterized by a set of associated symptoms.”47 These subtle differences in language have important clinical implications (see chapter 1 in Jennett B, “The Vegetative State: Medical Facts, Ethical and Legal Dilemmas,” for further discussion).48
In 2010, the European Task Force on DoC suggested that the term, VS, be replaced with unresponsive wakefulness syndrome (UWS).49 This recommendation was largely motivated by concerns that this term was pejorative and dehumanizing. A similar term, wakeful unconscious state, was considered by the Aspen Neurobehavioral Workgroup but was rejected for fear that it would cause confusion given the entrenchment of VS in existing diagnostic coding systems, registries, and databases. The Workgroup also argued that the term, “unresponsive,” does not imply unconsciousness–a core feature of VS. The continued use of 2 different terms for this syndrome, mostly along geographic lines, complicates communication between providers and public understanding of an already complex condition.
Minimally Conscious State
Prior to the introduction of the term MCS, patients with inconsistent or minimal signs of conscious awareness were lumped together with those in VS. The conflation of these 2 groups failed to recognize that early recovery of cognitively mediated behavior, even when minimal, is predictive of further functional recovery.50,51 The International Working Party on VS considered transitional vegetative state, inconsistent low awareness state, and consistent low awareness state, but none of these terms achieved broad acceptance.20 In 1995, the Brain Injury Interdisciplinary Special Interest Group of the ACRM introduced the term, minimally responsive state, to reflect evidence of inconsistent interaction with the environment.19 This term was eventually abandoned as it does not distinguish reflexive from voluntary or cognitively mediated behavior.
In 2002, the Aspen Neurobehavioral Workgroup developed a case definition for the minimally conscious state. The Workgroup defined a lower boundary to clearly differentiate MCS from VS, and an upper boundary to mark emergence from MCS. Because the case definition and accompanying diagnostic criteria (Box 3) were established by expert consensus, they met with considerable controversy.52,53 More recently, some experts have advocated for replacing MCS with cortically mediated state (CMS), arguing that CMS simply acknowledges the presence of isolated cortical connectivity, whereas consciousness requires sustained and complex integration of cortical networks.54 Others argue that MCS should not be replaced by CMS because it negates border between MCS and eMCS.55
Box 3. Diagnostic criteria of MCS.
To establish the diagnosis of MCS, at least one of the following behaviors must be demonstrated on a reproducible or sustained basis:
Following simple commands;
Gestural or verbal yes/no responses regardless of accuracy;
Intelligible verbalization;
- Purposeful behavior including movements or affective behaviors that occur in contingent relation to relevant environmental stimuli and are not due to reflexive activity, including:
- Vocalizations or gestures that occur in direct response to the linguistic content of questions
- Reaching for objects that demonstrates a clear relationship between object location and direction of reach
- Touching or holding objects in a manner that accommodates the size and shape of the object
- Pursuit eye movement or sustained fixation that occurs in direct response to moving or salient stimuli
- Appropriate smiling or crying in response to the linguistic or visual content of emotional but not to neutral topics or stimuli.42
The hallmark feature of MCS is behavioral fluctuation, which complicates diagnostic assessment and contributes to the 40% rate of misdiagnosis.4–6 It is also essential to be alert to common confounding factors such as sedating medications, occult illness, subclinical seizure, and unrecognized hydrocephalus.56 These problems can be mitigated to some extent by conducting serial assessments.57 Confounding conditions will be discussed in greater detail in articles six and seven on DoC assessment measures. Early detection of MCS is essential in view of its favorable prognostic implications and the potential harmful effects of misdiagnosis on family members.58
Minimally Conscious State Subtypes: Minimally Conscious State +/−
In 2011, in the Coma Science Group in Belgium proposed that MCS be subcategorized into MCS plus (MCS+) and MCS minus (MCS−) based on behavioral signs of preserved language function.59 Functional connectivity studies have shown that patients in MCS + have higher cerebral metabolism in left hemisphere cortical areas encompassing the language network, compared to patients with MCS−, who have demonstrably less connectivity between Broca’s region and language cortices.60 The MCS plus/minus distinction appears to be clinically relevant as patients in MCS + demonstrate less functional disability on standardized outcome measures.51,61 The 11th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-11) now includes MCS− and MCS + as diagnostic categories (Box 4), which may support population-based mechanistic studies of DoC.62
Box 4. Diagnostic criteria of MCS plus/minus.
The diagnosis of MCS plus requires at least one clearly discernible language sign, including:
Response to command;
Intelligible verbalization; or
Intentional communication (at least 2 instances of a verbal or gestural yes or no signal, regardless of accuracy).
The diagnosis of MCS minus requires at least one clearly discernible behavioral sign of awareness, including:
Visual pursuit or fixation;
Object localization;
Localization to noxious stimulation;
Object manipulation;
Automatic motor behavior.61
Minimally Conscious State Subtypes: Akinetic Mutism, Hyperkinetic Mutism
Akinetic mutism (AM) is characterized by spontaneous visual pursuit with minimal to no behavioral evidence of command following, vocalization, emotional expression, and other forms of goal-directed behavior.63–65 Increased behavioral activity may be prompted by exposure to high intensity stimuli or neurostimulants.66 The basis for the severely reduced level of behavioral activity in AM is the downregulation of frontal-subcortical circuits that regulate drive functions responsible for behavioral initiation and persistence.26 The “telephone effect” described by Fisher in 1983 illustrates the mechanism underlying AM.67 The patient initially presents as mute and motionless but when exposed to a ringing telephone, picks up the receiver and begins speaking fluently. The ringing phone represents a salient sensory cue that initiates a temporary reversal in depressed drive triggering the release of overlearned behaviors.67
AM is usually caused by the bilateral or orbito-basal cortex. Patients with AM appear attentive but exhibit little to no speech or movement14 despite maintaining intrinsic capability to move and speak.19,26,68 The lesion profile usually includes the involvement of the anterior cingulate bilaterally, medial frontal cortex, paramedian mesodiencephalon, globus pallidus, caudate nucleus and/or medial forebrain bundle.14,69 Hyperkinetic mutism is a related condition characterized by heightened vigilance and non-goal-directed motor activity.14 Bilateral temporal, parietal, and occipital junction lesions are presumed responsible for the ballistic, continuous and unrestrained movements.67,70,71
Differentiating Vegetative State and Minimally Conscious State from Locked-in Syndrome
The locked-in syndrome (LIS) is not a DoC, but may be misdiagnosed as VS or MCS due to the severely limited or total loss of speech and movement.72,73 LIS may arise from mass lesions, infection, trauma, or demyelinating disorders that affect the ventral pons or caudal ventral midbrain,73 resulting in quadriplegia, apnea, bulbar palsy (ie, anarthria and dysphagia), and sensory dysfunction.73,74 The distinguishing feature of LIS is the preservation of vertical eye movements and voluntary blinking as these behaviors allow affected patients to communicate.64,75,76 In rare cases, ocular mobility is also impaired, making it virtually impossible to distinguish from VS on bedside examination.
Emergence from Minimally Conscious State and the Posttraumatic Confusional State
The Aspen Neurobehavioral Workgroup proposed that functional communication and/or functional object should demarcate the upper boundary of MCS, signaling emergence from MCS (eMCS).42 Functional communication was defined as accurate verbal or gestural yes-no responses to six consecutive situational orientation questions (eg, “Am I touching my nose?”) on 2 consecutive examinations. Functional object use is demonstrated through generally appropriate use of at least 2 different objects on 2 consecutive evaluations. (eg, hairbrush, cup, pen). Both behaviors require the integration of multiple cortical networks to support the cognitive processes (eg, language comprehension, attention, motor control) that underlie them. Some have argued that functional communication should not be a criterion for eMCS as some patients who never experienced DoC also have difficulty meeting the threshold for functional communication.77,78 Recent evidence suggests that consistent command-following recovers at nearly the same time79 and is as difficulty to achieve80 as functional communication and functional object and, for these reasons, should also mark the transition to eMCS.
Work by neurologists C.P Symonds and W.R. Russell in the early-mid 1900’s observed that the period after sustaining a blow to the head is marked by a “clouded consciousness” and a constellation of symptoms, including profound disorientation in space and time, restlessness, perceptual disturbances, labile emotions, and so forth. The term they used to describe their observations was post-traumatic amnesia (PTA), which “is taken to end at the time from which the patient can give a clear and consecutive account of what was happening around him.”81
In the 1990’s, Stuss and colleagues82 proposed that post traumatic confusional state (PTCS) replace PTA based on observations that PTA was typically accompanied by distractibility, impaired judgement, perceptual disturbance, restlessness, sleep disorder, confabulation, and emotional or behavioral dysregulation, among other symptoms. In 2020, the American Congress of Rehabilitation Medicine Disorders of Consciousness Special Interest Group published a case definition for PTCS.83 This evidence-informed case definition characterizes patients meeting criteria for eMCS but with ongoing symptoms of confusion that impede functional independence, limit cooperation in rehabilitation, interfere with personal safety, and limit appropriate engagement with others and the environment. Nearly all patients emerging from MCS meet the criteria for PTCS based on the presence of ongoing cognitive impairment, disorientation, agitation, and symptom fluctuation (Box 5).50
Box 5. Core features to establish the diagnosis of PTCS.
All four of the following core features must be present to establish the diagnosis of PTCS:83
Disturbances of attention: Reduced ability to focus or sustain attention
Disorientation: Impaired orientation to place, time, and situation
Disturbances of memory: Impaired ability to encode and recall new information
Fluctuation: The character and severity of the disturbance waxes and wanes during the course of the day
In addition to the four core features, PTCS can include any of the following:
Emotional and/or behavioral disturbances: Including but not limited to agitation/restlessness and/or hypoactivity; irritability, impulsivity, disinhibition, aggression and/or decreased responsiveness; affective lability and/or flattening
Sleep-wake cycle disturbance: Excessive sleep, insufficient sleep, alteration of normal sleep pattern, or decreased level of arousal
Delusions: Fixed false beliefs
Perceptual disturbance: Illusions, hallucinations
Confabulation: False memory
To meet the criteria for recovery from PTCS, patients must demonstrate significant improvement in all core features and 5 associated features of PTCS such that deficits in attention, orientation, memory, and behavioral consistency no longer have a major impact on the patient’s functional independence for basic self-care and safety awareness. Most patients eventually recover from PTCS, though ongoing assistance is typically still required to meet basic needs.50 No single measure assesses all symptoms of confusion, but the Confusion Assessment Protocol,84 a multidimensional tool derived from previously validated measures, addresses most aspects of PTCS.
Although similarities in the symptom profiles associated with PTCS and delirium have been noted,85–88 for patients with DoC PTCS is considered a positive prognostic indicator and an expected milestone of ongoing recovery. Conversely, delirium is associated with a decline in function and a worse prognosis.89–91 Moreover, the pathophysiological mechanisms resulting in PTCS are likely to differ significantly compared to those underlying other causes of delirium such as infection and anesthesia.92 Consistent use of the term PTCS to characterize patients who demonstrate the symptoms described above is necessary to support research and clinical practice specific to the effects of brain injury. For example, given that PTCS has been studied primary in TBI, whether the results of prior studies can be applied to non-traumatic etiologies (eg, cardiac arrest) is an area requiring further study.
Cognitive Motor Dissociation
The diagnostic accuracy of DoC has improved a significant with the development and dissemination of standardized assessments. However, the behavioral output required to clearly demonstrate awareness on these assessments may be masked by the disconnection of efferent pathways, peripheral injury, contracture, or hypotonicity, and other factors. Advanced neuroimaging and electrophysiological techniques, such as task-based functional magnetic resonance imaging (fMRI) and EEG assess consciousness by instructing patients to covertly follow a command (eg, imagine opening and closing your hand) while recording signals from the brain. Volitional brain activity is measured during the task and compared to resting brain activity levels.
Cognitive motor dissociation (CMD), or covert consciousness, describes patients who lack behavioral signs of awareness (ie, diagnosis of coma, VS, MCS+) yet exhibit evidence of command-following on fMRI or EEG assessment.93 This phenomenon was first demonstrated using task-based fMRI to show the preservation of volitional brain activity and neural networks in patients who lack motoric output, self-expression, or appear unresponsive on bedside examination.94 Various terms have been used, such as functional locked-in syndrome,59 covert cognition,95 non-behavioral MCS,96 and CMD,8 to describe this distinct, subgroup of patients with DoC. CMD may be present in up to 15–20% of patients with subacute-to chronic DoC.97,98
There is also evidence suggesting that CMD may be present in as many 15% of patients during the acute period (ie, first 7 days post-onset), early detection of CMD is associated with recovery of at least partial functional independence at 1-year post injury99 and favorable recovery occurs earlier in patients with CMD as compared to those without CMD.100
The term, covert cortical processing (CCP), has recently been proposed to describe a patients with DoC who do not show evidence of language comprehension on behavioral examination (eg, MCS−), but exhibit fMRI or EEG responses to spoken words or phrases.93,101–106 Although CCP cannot provide definitive evidence of volitional cognitive processing, it may be a useful predictor of ongoing recovery.107
The 2018 AAN-ACRM-NIDILRR and the 2020 European Academy of Neurology DoC guidelines both recommend the use of advanced neuroimaging and EEG methods to assess for CMD in some circumstances.9,10 These approaches are currently only available in select academic centers and their utility across healthcare systems is still being debated.11,108,109 Nevertheless, when complemented by standardized behavioral evaluation, the direct assessment of consciousness via fMRI and EEG, has the potential to fundamentally change diagnosis, prognosis, and treatment for patients with DoC.101
SUMMARY
DoC taxonomy has progressed over the last 50 years as multidisciplinary practice guidelines have improved the consistency of nomenclature and diagnostic criteria. Recent work by the Neurocritical Care Society’s Curing Coma Campaign provides an excellent example of international and interprofessional collaboration making strides toward clarity of diagnostic criteria and operational definitions within coma research. However, global consensus has not yet been achieved, compromising communication between clinicians and consumers, hindering epidemiologic studies, and complicating outcomes research. Future efforts should aim to establish a more rational DoC taxonomy that integrates behavioral and pathophysiologic characteristics.
CLINICS CARE POINTS.
Clinicians should adhere to evidence-based practice guidelines, such as those published by AAN-ACRM-NIDILLR in 2018, when conducting diagnostic assessment in patients with DoC.
Despite advancements in DoC taxonomy, areas of ambiguity and disagreement persist, compromising diagnostic accuracy, prognostication, and family/caregiver counseling.
Global interdisciplinary initiatives such as the Curing Coma Campaign provide an unprecedented opportunity for clinicians and investigators to establish uniform guidelines for a rational DoC taxonomy.
KEY POINTS.
Recent practice guidelines for the management of patients with disorders of consciousness (DoC) published in the U.S., Europe, and U.K. include evidence-based definitions, diagnostic criteria and assessment methods, improving consistency in terminology and approach to classification.
While behavioral assessment remains the gold standard for the detection of consciousness and differential diagnosis among DoC, the syndrome of cognitive motor dissociation (ie, “covert consciousness”) serves as a reminder that behavior is a weak proxy for consciousness.
A more rational taxonomy that considers behavioral characteristics, as well as underlying pathophysiologic features, is needed to improve DoC classification and treatment precision.
ACKNOWLEDGEMENTS
The contents of this manuscript were developed with support from the National Institute on Disability, Independent Living, and Rehabilitation Research (NIDILRR grant numbers: 90DPTB0011, 90DPTB0027). NIDILRR is a Center within the United States Administration for Community Living (ACL), Department of Health and Human Services (HHS) and the VA TBI Model System. The contents of this publication do not necessarily represent the policy of NIDILRR, ACL, HHS, and you should not assume endorsement by the United States Federal Government.
Footnotes
DISCLOSURE
No disclosures.
REFERENCES
- 1.Young MJ, Bodien YG, Giacino JT, et al. The neuroethics of disorders of consciousness: a brief history of evolving ideas. Brain 2021;144(11):3291–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polansky R, Arstotle’s de Anima: A Critical Commentary. Cambridge University Press, 2007. Book 1 of Aristotle’s de Anima. [Google Scholar]
- 3.Plum F, Posner JB. The diagnosis of stupor and coma. Contemp Neurol Ser 1972;10:1–286. [PubMed] [Google Scholar]
- 4.Andrews K, Murphy L, Munday R, et al. Misdiagnosis of the vegetative state: retrospective study in a rehabilitation unit. BMJ 1996;313(7048):13–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schnakers C, Vanhaudenhuyse A, Giacino J, et al. Diagnostic accuracy of the vegetative and minimally conscious state: clinical consensus versus standardized neurobehavioral assessment. BMC Neurol 2009;9(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Childs NL, Mercer WN, Childs HW. Accuracy of diagnosis of persistent vegetative state. Neurology 1993;43(8):1465. [DOI] [PubMed] [Google Scholar]
- 7.Reed GM. Toward ICD-11: Improving the clinical utility of WHO’s international classification of mental disorders. Prof Psychol Res Pr 2010;41(6):457–64. [Google Scholar]
- 8.Schiff ND. Cognitive motor dissociation following severe brain injuries. JAMA Neurol 2015;72(12):1413. [DOI] [PubMed] [Google Scholar]
- 9.Giacino JT, Katz DI, Schiff ND, et al. Practice guideline update recommendations summary: disorders of consciousness: report of the guideline development, dissemination, and implementation subcommittee of the American academy of neurology; the American congress of rehabilitation medicine; and the national institute on disability, independent living, and rehabilitation research. Neurology 2018;91(10):450–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kondziella D, Bender A, Diserens K, et al. European academy of neurology guideline on the diagnosis of coma and other disorders of consciousness. Eur J Neurol 2020;27(5):741–56. [DOI] [PubMed] [Google Scholar]
- 11.Royal College of Physicians. Prolonged disorders of consciousness following sudden onset brain injury: National clinical guidelines. London: RCP; 2020. [Google Scholar]
- 12.Laureys S, Schiff ND. Coma and consciousness: paradigms (re)framed by neuroimaging. Neuroimage 2012;61(2):478–91. [DOI] [PubMed] [Google Scholar]
- 13.Posner JB, Saper CB, Schiff ND, Plum F. Pathophysiology of signs and symptoms of coma. In: Posner JB, Saper CB, Schiff ND, Plum F, editors. Plum and Posner’s diagnosis of stupor and coma. 4th ed. New York, NY: Oxford University Press; 2007. p. 3–37. [Google Scholar]
- 14.Schiff ND, Plum F. The role of arousal and “gating” systems in the neurology of impaired consciousness. J Clin Neurophysiol 2000;17(5):438–52. [DOI] [PubMed] [Google Scholar]
- 15.Kinomura S, Larsson J, Gulyás B, et al. Activation by attention of the human reticular formation and thalamic intralaminar nuclei. Science 1996;271(5248): 512–5. [DOI] [PubMed] [Google Scholar]
- 16.Schiff ND. Central thalamic deep-brain stimulation in the severely injured brain: rationale and proposed mechanisms of action. Ann N Y Acad Sci 2009;1157(1): 101–16. [DOI] [PubMed] [Google Scholar]
- 17.Medical aspects of the persistent vegetative state. N Engl J Med 1994;330(21): 1499–508. [DOI] [PubMed] [Google Scholar]
- 18.Medical aspects of the persistent vegetative state. N Engl J Med 1994;330(22): 1572–9. [DOI] [PubMed] [Google Scholar]
- 19.American Congress of Rehabilitation Medicine. Recommendations for use of uniform nomenclature pertinent to patients with severe alterations in consciousness. Arch Phys Med Rehabil 1995;76(2):205–9. [DOI] [PubMed] [Google Scholar]
- 20.Andrews K International working party on the management of the vegetative state: summary report. Brain Inj 1996;10(11):797–806. [DOI] [PubMed] [Google Scholar]
- 21.Giacino J, Whyte J. The vegetative and minimally conscious states: current knowledge and remaining questions. J Head Trauma Rehabil 2005;20(1):30–50. [DOI] [PubMed] [Google Scholar]
- 22.Giacino JT, Katz DI, Schiff ND, et al. Practice guideline update recommendations summary: disorders of consciousness. Arch Phys Med Rehabil 2018; 99(9):1699–709. [DOI] [PubMed] [Google Scholar]
- 23.Giacino JT, Bodien YG, Zuckerman D, et al. Empiricism and rights justify the allocation of health care resources to persons with disorders of consciousness. AJOB Neuroscience 2021;12(2–3):169–71. [DOI] [PubMed] [Google Scholar]
- 24.Koehler PJ, Wijdicks EFM. Historical study of coma: looking back through medical and neurological texts. Brain 2008;131(3):877–89. [DOI] [PubMed] [Google Scholar]
- 25.Kretschmer E Das apallische Syndrom. Z f d g Neur u Psych 1940;169(1): 576–9. [Google Scholar]
- 26.Giacino JT, Fins JJ, Laureys S, et al. Disorders of consciousness after acquired brain injury: the state of the science. Nat Rev Neurol 2014;10(2):99–114. [DOI] [PubMed] [Google Scholar]
- 27.Fischer DB, Boes AD, Demertzi A, et al. A human brain network derived from coma-causing brainstem lesions. Neurology 2016;87(23):2427–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Provencio JJ, Hemphill JC, Claassen J, et al. The curing coma campaign: framing initial scientific challenges—proceedings of the first curing coma campaign scientific advisory council meeting. Neurocritical Care 2020; 33(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Helbok R, Rass V, Beghi E, et al. The curing coma campaign international survey on coma epidemiology, evaluation, and therapy (COME TOGETHER). Neurocritical Care 2022;37(1):47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kondziella D, Frontera JA. Pearls & Oy-sters: eyes-open coma. Neurology 2021; 96(18):864–7. [DOI] [PubMed] [Google Scholar]
- 31.Kondziella D, Amiri M, Othman MH, et al. Incidence and prevalence of coma in the UK and the USA. Brain Communications 2022;4(5):fcac188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olson DM, Hemphill JC, The Curing Coma Campaign and its Executive Committe. The curing coma campaign: challenging the paradigm for disorders of consciousness. Neurocritical Care 2021;35(Suppl 1):1–3. [DOI] [PubMed] [Google Scholar]
- 33.Claassen J, Akbari Y, Alexander S, et al. Proceedings of the first curing coma campaign NIH symposium: challenging the future of research for coma and disorders of consciousness. Neurocritical Care 2021;35(S1):4–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adams ZM, Fins JJ. The historical origins of the vegetative state: received wisdom and the utility of the text. J Hist Neurosci 2017;26(2):140–53. [DOI] [PubMed] [Google Scholar]
- 35.Bichat X Recherches physiologiques sur la vie et la mort. Translated by Watkins Tobias. Philadelphia: Smith and Maxwell; 1809. [Google Scholar]
- 36.Timme W, Davis TK and Riley HA, The vegetative nervous system: an investigation of the most recent answers, In: Proceedings of the association of nervous and mental diseases, 1928, 3–11, Williams & Wilkins; Baltimore, MD. [Google Scholar]
- 37.Plum F, Schiff N, Ribary U, et al. The American Association for Research into Nervous and Mental Diseases, Coordinated expression in chronically unconscious persons. Phil Trans Roy Soc Lond B 1998;353(1377):1929–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arnould M, Vigouroux R, Vigouroux M. Etats frontiers entre la vie et la mort en neuron-tramatologie. Neurochirurgica (Stuttg) 1963;(6):1–21. [Google Scholar]
- 39.Vapalahti M, Troupp H. Prognosis for patients with severe brain injuries. BMJ 1971;3(5771):404–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jennett B, Plum F. Persistent vegetative state after brain damage. Lancet 1972; 299(7753):734–7. [DOI] [PubMed] [Google Scholar]
- 41.Practice parameters [RETIRED]: assessment and management of patients in the persistent vegetative state (Summary statement). Neurology 1995;45(5): 1015–8. [DOI] [PubMed] [Google Scholar]
- 42.Giacino JT, Ashwal S, Childs N, et al. The minimally conscious state: definition and diagnostic criteria. Neurology 2002;58(3):349–53. [DOI] [PubMed] [Google Scholar]
- 43.Giacino JT, Zasler ND, Katz DI, et al. Development of practice guidelines for assessment and management of the vegetative and minimally conscious states. J Head Trauma Rehabil 1997;12(4):79–89. [Google Scholar]
- 44.Giacino J, Kalmar K. Diagnostic and prognostic guidelines for the vegwive and minimally conscious states. Neuropsychol Rehabil 2005;15(3–4):166–74. [DOI] [PubMed] [Google Scholar]
- 45.Estraneo A, Moretta P, Loreto V, et al. Late recovery after traumatic, anoxic, or hemorrhagic long-lasting vegetative state. Neurology 2010;75(3):239–45. [DOI] [PubMed] [Google Scholar]
- 46.Definition of state noun from the Oxford Advanced American Dictionary. Published online 2023. Available at: https://www.oxfordlearnersdictionaries.com/us/definition/american_english/state_1. Accessed May 1, 2023.
- 47.“Syndrome.” Oxford Advanced American Dictionary. Published online 2023. Available at: https://www.oxfordlearnersdictionaries.com/us/definition/american_english/syndrome#:~:text=syndrome-,noun,is%20associated%20with%20frequent%20coughing. Accessed May 1, 2023.
- 48.Howard RS. The Vegetative state: Medical Facts, Ethical and Legal Dilemmas. Brain 2002;125(12):2782. [Google Scholar]
- 49.Laureys S, Celesia GG, Cohadon F, et al. , the European Task Force on Disorders of Consciousness, Unresponsive wakefulness syndrome: a new name for the vegetative state or apallic syndrome, BMC Med, 8(1), 2010, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bodien YG, Martens G, Ostrow J, et al. Cognitive impairment, clinical symptoms and functional disability in patients emerging from the minimally conscious state. NRE 2020;46(1):65–74. [DOI] [PubMed] [Google Scholar]
- 51.Giacino JT, Sherer M, Christoforou A, et al. Behavioral recovery and early decision making in patients with prolonged disturbance in consciousness after traumatic brain injury. J Neurotrauma 2020;37(2):357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shewmon DA. The minimally conscious state: definition and diagnostic criteria. Neurology 2002;58(3):506 [author reply: 506–507]. [PubMed] [Google Scholar]
- 53.Coleman D The minimally conscious state: definition and diagnostic criteria. Neurology 2002;58(3):506 [author reply: 506–507]. [PubMed] [Google Scholar]
- 54.Naccache L Minimally conscious state or cortically mediated state? Brain 2018; 141(4):949–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bayne T, Hohwy J, Owen AM. Reforming the taxonomy in disorders of consciousness. Ann Neurol 2017;82(6):866–72. [DOI] [PubMed] [Google Scholar]
- 56.Whyte J, Nordenbo AM, Kalmar K, et al. Medical complications during inpatient rehabilitation among patients with traumatic disorders of consciousness. Arch Phys Med Rehabil 2013;94(10):1877–83. [DOI] [PubMed] [Google Scholar]
- 57.Wannez S, Heine L, Thonnard M, et al. , Coma Science Group collaborators. The repetition of behavioral assessments in diagnosis of disorders of consciousness. Ann Neurol 2017;81(6):883–9. [DOI] [PubMed] [Google Scholar]
- 58.Demertzi A, Antonopoulos G, Heine L, et al. Intrinsic functional connectivity differentiates minimally conscious from unresponsive patients. Brain 2015;138(9): 2619–31. [DOI] [PubMed] [Google Scholar]
- 59.Bruno MA, Vanhaudenhuyse A, Thibaut A, et al. From unresponsive wakefulness to minimally conscious PLUS and functional locked-in syndromes: recent advances in our understanding of disorders of consciousness. J Neurol 2011; 258(7):1373–84. [DOI] [PubMed] [Google Scholar]
- 60.Bruno MA, Majerus S, Boly M, et al. Functional neuroanatomy underlying the clinical subcategorization of minimally conscious state patients. J Neurol 2012;259(6):1087–98. [DOI] [PubMed] [Google Scholar]
- 61.Thibaut A, Bodien YG, Laureys S, et al. Minimally conscious state “plus”: diagnostic criteria and relation to functional recovery. J Neurol 2020;267(5):1245–54. [DOI] [PubMed] [Google Scholar]
- 62.World Health Organization (WHO). International Classification of Diseases, Eleventh Revision (ICD-11). Published online 2021. 2019 Licensed under Creative Commons Attribution-NoDerivatives 3.0 IGO license (CC BY-ND 3.0 IGO). Available at: https://icd.who.int/browse. Accessed May 1, 2023.
- 63.Cairns H, Oldfield RC, Pennybacker JB, et al. Akinetic mutism with an epidermoid cyst of the 3rd ventricle. Brain 1941;64(4):273–90. [Google Scholar]
- 64.Giacino J Disorders of consciousness: differential diagnosis and neuropathologic features. Semin Neurol 1997;17(02):105–11. [DOI] [PubMed] [Google Scholar]
- 65.Formisano R, D’Ippolito M, Risetti M, et al. Vegetative state, minimally conscious state, akinetic mutism and Parkinsonism as a continuum of recovery from disorders of consciousness: an exploratory and preliminary study. Funct Neurol 2011;26(1):15–24. [PMC free article] [PubMed] [Google Scholar]
- 66.Nagaratnam N, Nagaratnam K, Ng K, et al. Akinetic mutism following stroke. J Clin Neurosci 2004;11(1):25–30. [DOI] [PubMed] [Google Scholar]
- 67.Fisher MC. Honored guest presentation: abulia minor vs. Agitated behavior. Neurosurgery 1984;31(Supplement 1):9–31. [DOI] [PubMed] [Google Scholar]
- 68.Tibbetts PE. The anterior cingulate cortex, akinetic mutism, and human volition. Brain Mind 2001;2(3):323–41. [Google Scholar]
- 69.Royal College of Physicians. Prolonged disorders of consciousness: national clinical guidelines. London: RCP; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Inbody S, Jankovic J. Hyperkinetic mutism. Neurology 1987;37(9):1566. [DOI] [PubMed] [Google Scholar]
- 71.Hesselink JMK, van Gijn J, Verwey JC. Hyperkinetic mutism. Neurology 1987; 37(9):1566. [DOI] [PubMed] [Google Scholar]
- 72.Laureys S, Pellas F, Van Eeckhout P, et al. The locked-in syndrome : what is it like to be conscious but paralyzed and voiceless? Prog Brain Res 2005;150: 495–611. Elsevier. [DOI] [PubMed] [Google Scholar]
- 73.M Das J, Anosike K, Asuncion RMD. Locked-in Syndrome. In: StatPearls. StatPearls Publishing; 2022. Available at: http://www.ncbi.nlm.nih.gov/books/NBK559026/. Accessed December 13, 2022. [PubMed] [Google Scholar]
- 74.Patterson JR, Grabois M. Locked-in syndrome: a review of 139 cases. Stroke 1986;17(4):758–64. [DOI] [PubMed] [Google Scholar]
- 75.Giacino JT, Schnakers C, Rodriguez-Moreno D, et al. Behavioral assessment in patients with disorders of consciousness: gold standard or fool’s gold? Prog Brain Res 2009;177:33–48. Elsevier. [DOI] [PubMed] [Google Scholar]
- 76.Rodriguez Moreno D, Schiff ND, Giacino J, et al. A network approach to assessing cognition in disorders of consciousness. Neurology 2010;75(21):1871–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nakase-Richardson R, Yablon SA, Sherer M, et al. Serial yes/no reliability after traumatic brain injury: implications regarding the operational criteria for emergence from the minimally conscious state. J Neurol Neurosurg Psychiatr 2008;79(2):216–8. [DOI] [PubMed] [Google Scholar]
- 78.Nakase-Richardson R, Yablon SA, Sherer M, et al. Emergence from minimally conscious state: Insights from evaluation of posttraumatic confusion. Neurology 2009;73(14):1120–6. [DOI] [PubMed] [Google Scholar]
- 79.Golden K, Erler KS, Wong J, et al. Should consistent command-following be added to the criteria for emergence from the minimally conscious state? Arch Phys Med Rehabil 2022;103(9):1870–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Weaver JA, Cogan AM, O’Brien KA, et al. Determining the hierarchy of coma recovery scale-revised rating scale categories and alignment with aspen consensus criteria for patients with brain injury: a rasch analysis. J Neurotrauma 2022;39(19–20):1417–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Symonds CP, Ritchie Russell W. Accidental head injuries. Lancet 1943; 241(6227):7–10. [Google Scholar]
- 82.Stuss DT, Binns MA, Carruth FG, et al. The acute period of recovery from traumatic brain injury: posttraumatic amnesia or posttraumatic confusional state? J Neurosurg 1999;90(4):635–43. [DOI] [PubMed] [Google Scholar]
- 83.Sherer M, Katz DI, Bodien YG, et al. Post-traumatic confusional state: a case definition and diagnostic criteria. Arch Phys Med Rehabil 2020;101(11): 2041–50. [DOI] [PubMed] [Google Scholar]
- 84.Sherer M, Nakase-Thompson R, Yablon SA, et al. Multidimensional assessment of acute confusion after traumatic brain injury. Arch Phys Med Rehabil 2005; 86(5):896–904. [DOI] [PubMed] [Google Scholar]
- 85.Slooter AJC, Otte WM, Devlin JW, et al. Updated nomenclature of delirium and acute encephalopathy: statement of ten Societies. Intensive Care Med 2020; 46(5):1020–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ropper AH, Samuels MA, Klein JP. Chapter 20. Delirium and other acute confusional states. In: Adams and victor’s principles of neurology, 10e. The McGrawHill Companies; 2014. Available at: accessmedicine.mhmedical.com/content.aspx?aid=57615881. Accessed December 13, 2022. [Google Scholar]
- 87.Morandi A, Pandharipande P, Trabucchi M, et al. Understanding international differences in terminology for delirium and other types of acute brain dysfunction in critically ill patients. Intensive Care Med 2008;34(10):1907–15. [DOI] [PubMed] [Google Scholar]
- 88.Disorders of attention: a frontier in neuropsychology. Phil Trans Roy Soc Lond B 1982;298(1089):173–85. [DOI] [PubMed] [Google Scholar]
- 89.Pompei P, Foreman M, Rudberg MA, et al. Delirium in hospitalized older persons: outcomes and predictors. J Am Geriatr Soc 1994;42(8):809–15. [DOI] [PubMed] [Google Scholar]
- 90.Brooks PB. Postoperative delirium in elderly patients. AJN, American Journal of Nursing 2012;112(9):38–49. [DOI] [PubMed] [Google Scholar]
- 91.Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med 2013;369(14):1306–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sherer M, Katz DI, Bodien YG. Seeking clarity about confusion. Arch Phys Med Rehabil 2021;102(2):339–40. [DOI] [PubMed] [Google Scholar]
- 93.Edlow BL, Claassen J, Schiff ND, et al. Recovery from disorders of consciousness: mechanisms, prognosis and emerging therapies. Nat Rev Neurol 2021; 17(3):135–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Owen AM, Coleman MR, Boly M, et al. Detecting awareness in the vegetative state. Science 2006;313(5792):1402. [DOI] [PubMed] [Google Scholar]
- 95.Schnakers C, Giacino JT, Løvstad M, et al. Preserved covert cognition in noncommunicative patients with severe brain injury? Neurorehabil Neural Repair 2015;29(4):308–17. [DOI] [PubMed] [Google Scholar]
- 96.Gosseries O, Zasler ND, Laureys S. Recent advances in disorders of consciousness: focus on the diagnosis. Brain Inj 2014;28(9):1141–50. [DOI] [PubMed] [Google Scholar]
- 97.Kondziella D, Friberg CK, Frokjaer VG, et al. Preserved consciousness in vegetative and minimal conscious states: systematic review and meta-analysis. J Neurol Neurosurg Psychiatr 2016;87(5):485–92. [DOI] [PubMed] [Google Scholar]
- 98.Schnakers C, Hirsch M, Noé E, et al. Covert cognition in disorders of consciousness: a meta-analysis. Brain Sci 2020;10(12):930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Claassen J, Doyle K, Matory A, et al. Detection of brain activation in unresponsive patients with acute brain injury. N Engl J Med 2019;380(26):2497–505. [DOI] [PubMed] [Google Scholar]
- 100.Egbebike J, Shen Q, Doyle K, et al. Cognitive-motor dissociation and time to functional recovery in patients with acute brain injury in the USA: a prospective observational cohort study. Lancet Neurol 2022;21(8):704–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Edlow BL, Chatelle C, Spencer CA, et al. Early detection of consciousness in patients with acute severe traumatic brain injury. Brain 2017;140(9):2399–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Menon D, Owen A, Williams E, et al. Cortical processing in persistent vegetative state. Lancet 1998;352(9123):200. [DOI] [PubMed] [Google Scholar]
- 103.Schiff ND, Plum F. Cortical function in the persistent vegetative state. Trends Cogn Sci 1999;3(2):43–4. [DOI] [PubMed] [Google Scholar]
- 104.Coleman MR, Davis MH, Rodd JM, et al. Towards the routine use of brain imaging to aid the clinical diagnosis of disorders of consciousness. Brain 2009; 132(9):2541–52. [DOI] [PubMed] [Google Scholar]
- 105.Fernández-Espejo D, Junqué C, Vendrell P, et al. Cerebral response to speech in vegetative and minimally conscious states after traumatic brain injury. Brain Inj 2008;22(11):882–90. [DOI] [PubMed] [Google Scholar]
- 106.Di HB, Yu SM, Weng XC, et al. Cerebral response to patient’s own name in the vegetative and minimally conscious states. Neurology 2007;68(12):895–9. [DOI] [PubMed] [Google Scholar]
- 107.Sokoliuk R, Degano G, Banellis L, et al. Covert speech comprehension predicts recovery from acute unresponsive states. Ann Neurol 2021;89(4):646–56. [DOI] [PubMed] [Google Scholar]
- 108.Wade DT, Turner-Stokes L, Playford ED, et al. Prolonged disorders of consciousness: a response to a “critical evaluation of the new UK guidelines”. Clin Rehabil 2022;36(9):1267–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Scolding N, Owen AM, Keown J. Prolonged disorders of consciousness: a critical evaluation of the new UK guidelines. Brain 2021;144(6):1655–60. [DOI] [PMC free article] [PubMed] [Google Scholar]


