Abstract
Background
Cutaneous infections caused by non-tuberculous mycobacteria (NTM) are extremely rare, particularly when they are localized to the facial area. This condition presents significant diagnostic challenges due to its unusual presentation and the need for precise microbiological identification.
Case Presentation
A two-year-old male patient presented with a progressively enlarging reddish-brown mass on the left side of his face. Despite the absence of systemic symptoms, the lesion’s growth warranted investigation due to its growth. Ultrasonography showed a hypoechoic mass in the dermis, indicating an underlying abscess. The subsequent aspiration resulted in pale yellow pus, which upon testing and culture, confirmed the presence of Mycobacterium avium complex infection, a species of NTM. This case exemplifies the synergy between imaging modalities and microbiological analysis, highlighting the crucial role of both in achieving favorable clinical outcomes in patients with suspected cutaneous NTM infections. Ultrasound can expedite diagnosis, improve treatment planning, and enhance patient care by enabling targeted interventions and monitoring response to therapy in these scenarios. However, it is the combination of pathogen-specific diagnostics that ensures accurate etiological attribution and appropriate antimicrobial stewardship.
Conclusion
Although rare, facial cutaneous infections caused by NTM still deserve thorough investigation to determine the exact cause. Ultrasound is used to identify cutaneous lesions, measure their extent, and guide surgical procedures. The ultimate diagnosis is based on microbiological confirmation.
Keywords: Cutaneous infections, Facial, Non-tuberculous mycobacteria (NTM), Case report
Background
Non-tuberculous mycobacteria (NTM) is a general term for a large group of mycobacteria except Mycobacterium tuberculosis complex and Mycobacterium leprae [1]. Most of them are parasitic bacteria, and only a few of them are pathogenic to humans, which belong to opportunistic pathogens [2]. NTM disease refers to human infection with NTM, which causes lesions in related tissues and organs. In recent years, NTM disease has increased rapidly and has become one of the important public health problems threatening human health [3]. However, cutaneous infections triggered by NTM are very rare, especially in the face. We report a case of facial cutaneous infection in a child triggered by NTM.
Case presentation
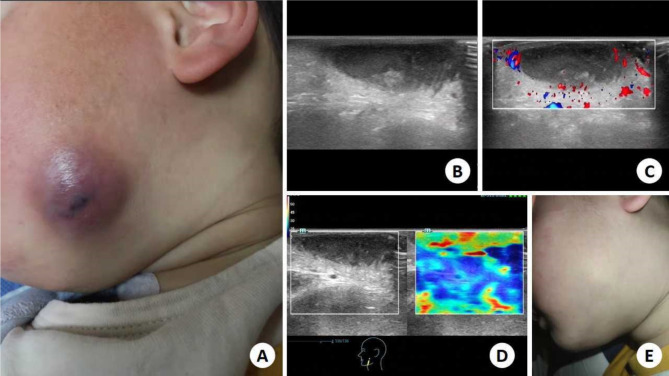
A 2-year-old boy was referred to the infection department. On the left side of the face, there is a reddish-brown round mass approximately 2 cm in diameter, characterized by well-defined borders and a smooth surface that exhibits no signs of collapse. The texture is soft with mild tenderness. (Fig. 1A). The patient’s father reported that eczema had developed a month ago, with a small erythema on the left face and was not treated in any way. Over time, erythema grows larger, shows no signs of improvement, highlights on the cutaneous surface and becomes darker. The patient had no symptoms, including fever, dyspnea, and chest pain, and they deny having any history of trauma and surgery. Subsequently, the patient underwent ultrasonography: a hypoechoic mass of about 2.9 × 0.6 cm appeared in the left facial dermis, with clear boundaries and uneven internal echo (Fig. 1B). A slight blood flow signal around the mass was visualised by Color doppler ultrasonography (Fig. 1C). Elastography ultrasonography showed that the mass was soft (Fig. 1D). Indicates the presence of thick internal pus. Then, 0.6 ml of pale yellow pus was aspirated from within the mass using a 5 ml syringe. The samples were tested and cultured. The pus samples were detected by the specificity of NTM homologous DNA sequence method, and the final result was Mycobacterium avium complex (MAC) infection, a nontuberculous mycobacterium (NTM). The patient received started treatment for the treatment of Mycobacterium avium intercellular infection (Azithromycin, 200 mg QD; Ethambutol, 200 mg QD; Rifabutin, 50 mg QD). Following four months of anti-infective therapy, the facial lesion demonstrated significant improvement, with the cutaneous color of the lesion returning to a state that was no longer markedly distinct from the surrounding tissue; however, minimal scarring remained visible at the site. Subsequently, the patient ceased the administration of anti-infective medications. During a follow-up visit two years post-treatment, the facial lesion had fully resolved, leaving only subtle scarring that was not readily apparent(Fig. 1E). According to the patient’s father, there were no significant residual marks present on the patient’s face, and previous concerns regarding cosmetic outcomes proved to be unfounded. The patient expressed high satisfaction with the current condition.
Fig. 1.
A 2-year-old boy was treated for a facial mass. A: Reddish-brown mass was visible on the left side of the face, highlighting the skin surface; B: Gray scale ultrasonography indicates a hypoechoic mass of about 2.9 × 0.6 cm appeared in the left facial dermis, with clear boundaries and uneven internal echo; C: Color Doppler Ultrasonography shows that the internal blood flow signal is not rich; D: Easastography ultrasonography showed that the mass was soft; E: After 2 years, the patient’s cutaneous had recovered and tiny scars were found
Discussion and conclusions
Cutaneous infected by NTM is very rare and difficult to diagnose. NTM includes more than 160 ubiquitous environmental acid fast staining bacterial species, some of which may cause human diseases [4]. Epidemiological studies of NTM disease pose significant challenges, making it difficult to obtain precise data on the incidence and prevalence across different countries or regions. This is primarily due to the fact that NTM disease reporting is not mandatory in most countries [3, 5]. Additionally, the accurate identification of NTM infections and the assessment of associated morbidity require specialized diagnostic methods, which can be technically demanding. Furthermore, the reported incidence and prevalence of NTM infections vary considerably among studies, reflecting regional differences in environmental exposures, diagnostic practices, and patient populations [6].
The host and pathogen factors leading to NTM infection are unclear. Traditional detection methods (supplemented by biochemical test after culture medium) can not provide reference for clinical diagnosis accurately and timely [7]. With the rapid development of molecular biology technology, strains can be quickly identified by using the specificity of NTM homologous DNA sequences (such as 16 S ribosomal DNA, ribosomal protein S1, inter transcriptional spacer 1, heat shock protein 65, rpoB, etc.). To identify the specific type of NTM causing the infection, we employed a sequence-based detection method targeting conserved regions unique to NTM. Clinical samples were collected under sterile conditions, and genomic DNA was extracted using a commercial kit. Specific primer pairs were designed to amplify the hsp65 gene, a highly conserved region among NTM species. PCR amplification was performed under optimized conditions, followed by agarose gel electrophoresis to confirm the presence of the expected amplicon sizes. Sequencing of the purified PCR products was conducted using Sanger sequencing technology. Sequence data were analyzed using NCBI’s BLAST tools against a database of known NTM sequences to identify the exact species [8]. This approach facilitated the precise diagnosis of the infection caused by Mycobacterium avium complex.
Ultrasound imaging in cutaneous infections demonstrates multifaceted utility, significantly contributing to diagnosis, treatment monitoring, and complication assessment [9]. As an adjunct diagnostic modality, it proficiently identifies tuberculous lesions within subcutaneous and deeper tissues, elucidating their dimensions, depth, structural characteristics, and the reactive changes in adjacent tissues, thereby facilitating diagnosis, particularly in instances such as scrofuloderma. By dynamically surveilling disease progression, ultrasound aids in evaluating therapeutic outcomes through tracking lesion modifications and structural improvements [10]. Moreover, it plays a pivotal role in guiding fine-needle aspirations or biopsies under precise sonographic guidance, enhancing the accuracy of specimen collection while minimizing procedural complications. Its capacity to detect associated complications, including lymphadenopathy, abscess formation, and vascular involvement, furnishes vital insights for devising comprehensive management strategies. Characterized by its non-invasive, convenient, and highly reproducible nature, ultrasound examination constitutes an indispensable component in the longitudinal surveillance and patient management of cutaneous tuberculosis, significantly enhancing therapeutic efficacy and ensuring patient safety [11].
Nontuberculous mycobacteria generally have higher rates of drug resistance and are treated for a longer period of time than tuberculous mycobacteria [1, 12]. Mycobacterium avium complex (MAC) predominantly impacts individuals with compromised immune systems. Commonly employed antibiotics in the treatment regimen include macrolides such as clarithromycin or azithromycin, alongside rifampicin and ethambutol [13]. Therapeutic protocols typically incorporate a combination of three or more agents to mitigate the development of bacterial resistance [14, 15]. When formulating a therapy plan for NTM disease, drugs should be selected according to the drug sensitivity test results of these drugs [4]. Actively monitor and manage drug safety and timely detect and deal with adverse reactions to anti-NTM drugs. Meanwhile, strategies for monitoring drug safety and managing adverse reactions during treatment are of paramount importance [16].
Future investigations should prioritise elucidating the mechanisms of NTM infections, with a particular focus on the host-pathogen interaction dynamics, to uncover novel targets for prevention and intervention [17, 18]. Concurrently, there is a pressing need to expedite the advancement of innovative, rapid diagnostic methodologies, including genomic-based approaches, to facilitate early and accurate NTM identification, thereby enhancing patient prognosis [1, 13].
In conclusion, while facial cutaneous infections caused by NTM are indeed less common, they nonetheless warrant a thorough investigation to ascertain the precise etiology. Ultrasound facilitates the characterization of cutaneous lesions, delineates their extent, and guides interventional procedures. However, the ultimate diagnosis hinges on microbiological confirmation.
Acknowledgements
The author thanked many additional members of our hospital department of ultrasound teams who helped with enrollment and evaluation of participants, and finally, the participants themselves.
Abbreviations
- NTM
Non-tuberculous mycobacteria
- MAC
Mycobacterium avium complex
Author contributions
Z.X.: data collation, paper writing, paper review, and fund support; D.L., C.F.W.: data collation and paper review.
Funding
This work was supported by funding from the Joint Pre-research Foundation of Zhejiang University(ZAYY229), and Hangzhou Medical and health science and technology plan project (A20220044, A20240074). The manuscript is not being considered for publication elsewhere. Written consent for publication was obtained from the patient’s father.
Data availability
The data that support the conclusions of this article are included in this published article.
Declarations
Ethics approval
The study was designed and conducted following the Helsinki Declaration and approved by the Ethics Committee of the Hangzhou Red Cross Hospital. An informed consent was signed by the patient as part of the hospital’s routine activity. The manuscript has been reviewed and approved by all named authors. The criteria for authorship have been met and each author believes that the manuscript represents honest work.
Consent for publication
The patient gave consent for publication.
Consent for publication
Written informed consent has been obtained from the patient’s legal guardian to publish this report and any accompanying images.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lin WH, Yao C, Mei L, Wang DP, Bao XD, Liu SS. Screening, epidemic trends and drug sensitivity analysis of nontuberculous mycobacteria in a local area of China. AM J TRANSL RES. 2024;16(7):3298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dahl VN, Pedersen AA, Norman A, Rasmussen EM, van Ingen J, Andersen AB, Wejse CM, Lillebaek T. Clinical significance, species distribution, and temporal trends of Nontuberculous Mycobacteria, Denmark, 1991–2022. EMERG INFECT DIS. 2024;30(9):1755–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu N, Li L, Wu S. Epidemiology and laboratory detection of non-tuberculous mycobacteria. HELIYON. 2024;10(15):e35311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kozińska M, Augustynowicz-Kopeć E, Gamian A, Chudzik A, Paściak M, Zdziarski P. Cutaneous and Pulmonary Tuberculosis-Diagnostic and Therapeutic Difficulties in a Patient with Autoimmunity. PATHOGENS 2023, 12(2). [DOI] [PMC free article] [PubMed]
- 5.Kim J, Hoon RJ, Jang J, Jang H, Lim JB. Comparative assessment of trough and peak levels and AUC24 for amikacin in nontuberculous mycobacterial infection. CLIN CHIM ACTA 2024:119963. [DOI] [PubMed]
- 6.Sreekumar A, Kumar A, Biswas R, Biswas L. Emerging and alternative strategies for the treatment of nontuberculous mycobacterial infections. EXPERT REV ANTI-INFE 2024:1–19. [DOI] [PubMed]
- 7.Kaul S, Jakhar D, Mehta S, Singal A. Cutaneous tuberculosis. Part II: complications, diagnostic workup, histopathologic features, and treatment. J AM ACAD DERMATOL. 2023;89(6):1107–19. [DOI] [PubMed] [Google Scholar]
- 8.Kong M, Li W, Kong Q, Dong H, Han A, Jiang L. Application of metagenomic next-generation sequencing in cutaneous tuberculosis. FRONT CELL INFECT MI. 2022;12:942073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hesselstrand R, Scheja A, Wildt M, Akesson A. High-frequency ultrasound of skin involvement in systemic sclerosis reflects oedema, extension and severity in early disease. Rheumatology. 2008;47(1):84–7. [DOI] [PubMed] [Google Scholar]
- 10.Catalano O, Roldán FA, Varelli C, Bard R, Corvino A, Wortsman X. Skin cancer: findings and role of high-resolution ultrasound. J ULTRASOUND. 2019;22(4):423–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia C, Wortsman X, Bazaes-Nuñez D, Pelizzari M, Gonzalez S, Cossio ML, De Barbieri F. Skin sonography in children: a review. PEDIATR RADIOL. 2022;52(9):1687–705. [DOI] [PubMed] [Google Scholar]
- 12.Wang XY, Jia QN, Li J, Zheng HY. Investigating cutaneous tuberculosis and nontuberculous mycobacterial infections a Department of Dermatology, Beijing, China: a comprehensive clinicopathological analysis. FRONT CELL INFECT MI. 2024;14:1451602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deshpande D, Magombedze G, Srivastava S, Gumbo T. Antibacterial action of penicillin against Mycobacterium avium complex. IJTLD Open. 2024;1(8):362–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morimoto K, Gallagher JR, Wagner D, Griffith DE, van Ingen J. Real-world patients’ diagnosis-to-treatment journey with Nontuberculous Mycobacterial Pulmonary Disease: a cross-sectional survey. INFECT DIS THER. 2024;13(8):1907–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conyers LE, Saunders BM. Treatment for non-tuberculous mycobacteria: challenges and prospects. FRONT MICROBIOL. 2024;15:1394220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Staden D, Haynes RK, Viljoen JM. Adapting clofazimine for treatment of cutaneous tuberculosis by using self-double-emulsifying drug Delivery systems. ANTIBIOTICS-BASEL 2022, 11(6). [DOI] [PMC free article] [PubMed]
- 17.Metersky ML, Fraulino D, Monday L, Chopra T. Current challenges in pulmonary nontuberculous mycobacterial infection: a case series with literature review. POSTGRAD MED 2024:1–12. [DOI] [PubMed]
- 18.Llerena C, Valbuena YA, Zabaleta AP, García AN. Prevalence of resistance to macrolides and aminoglycosides in Mycobacterium avium, M. Abscessus, and M. Chelonae identified in the Laboratorio Nacional De Referencia of Colombia from 2018 to 2022. BIOMEDICA. 2024;44(2):182–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the conclusions of this article are included in this published article.