The findings of this meta-analysis demonstrate that mind-body exercise positively influences bone mineral density, sleep quality, anxiety, depression, and fatigue among perimenopausal and postmenopausal women.
Overall, this meta-analysis of randomized controlled trials of mind-body exercise interventions for improving bone mineral density and menopausal symptoms in perimenopausal and postmenopausal women shows that mind-body exercise has a positive impact on bone mineral density, sleep quality, anxiety, depression, and fatigue in perimenopausal and postmenopausal women. Doctors can promote mind-body practice as an excellent non-drug treatment for managing menopausal symptoms.
Key Words: Menopause, Meta-analysis, Mind-body exercise, Randomized controlled trials
Abstract
Importance
The increasing attention to the management of perimenopausal and postmenopausal women parallels the growth of the aging population. Although hormone therapy is commonly used to alleviate menopausal symptoms, it carries a potential risk of cancer. Recently, mind-body exercises have emerged as innovative approaches for improving menopausal symptoms and bone health. However, research findings have needed to be more consistent, highlighting the significance of this study's systematic review of mind-body exercise effects on perimenopausal and postmenopausal women.
Objective
This study aims to evaluate the impact of mind-body exercises, including tai chi, yoga, Pilates, qigong, baduanjin, and mindfulness-based stress reduction, on bone mineral density, sleep quality, anxiety, depression, and fatigue among perimenopausal and postmenopausal women.
Evidence Review
Four electronic databases—PubMed, Embase, Cochrane Central Register of Controlled Trials, and Web of Science—were systematically searched from inception until July 2023. The search focused exclusively on randomized controlled trials to examine the impact of mind-body exercise interventions on perimenopausal and postmenopausal women. The methodological quality of the included studies was evaluated using the Cochrane Bias Risk Assessment tool.
Findings
A total of 11 randomized controlled trials, comprising 1,005 participants, were included in the analysis. Traditional meta-analysis indicated that mind-body exercise significantly enhanced bone mineral density in perimenopausal and postmenopausal women compared with control groups, with a standardized mean difference (SMD) of 0.41 (95% CI, 0.17 to 0.66; P = 0.001, I2 = 7%). In addition, significant improvements were observed in sleep quality (SMD, −0.48; 95% CI, −0.78 to −0.17; P = 0.002, I2 = 76%), anxiety reduction (SMD, −0.80; 95% CI, −1.23 to −0.38; P = 0.0002, I2 = 84%), depressive mood (SMD, −0.80; 95% CI, −1.17 to −0.44; P < 0.0001, I2 = 79%), and fatigue (SMD, −0.67; 95% CI, −0.97 to −0.37; P < 0.0001, I2 = 0%).
Conclusions and Relevance
The findings of this meta-analysis demonstrate that mind-body exercise positively influences bone mineral density, sleep quality, anxiety, depression, and fatigue among perimenopausal and postmenopausal women.
Key points
• Question: How do mind-body exercises impact bone mineral density and menopausal symptoms in women?
• Findings: This review included 11 randomized controlled trials encompassing 1,005 participants. The analysis revealed that mind-body exercises such as Pilates, yoga, tai chi, qigong, and mindfulness-based stress reduction, when compared with control groups, significantly improve bone mineral density, sleep quality, anxiety, depression, and fatigue in perimenopausal and postmenopausal women.
• Meaning: Mind-body exercises can be advocated as nonpharmacological interventions for managing menopausal symptoms, as a supplementary option alongside conventional practice.
According to the World Health Organization, the global population of postmenopausal women is projected to reach 1.2 billion by 2030 and over 1.6 billion by 2050.1,2 An increasing number of women are entering menopause.3,4 Sleep disorders, anxiety, depression, osteoporosis, and other menopausal symptoms, affecting 75% to 85% of this demographic, significantly impact their quality of life.5-7 Although hormone therapy has been a common approach to alleviate these symptoms, its potential carcinogenic risks8 have led to a growing trend among women seeking alternative treatments.9-11
In the realm of alternative treatments for menopause, mind-body exercises have gained popularity. Motorwala et al12 conducted a study on 30 postmenopausal women with osteoporosis, using yoga as an intervention. Their findings indicated an improvement in bone mineral density (BMD) among the participants. However, the study was limited by the absence of a control group and a small sample size.12 In another study, Newton et al13 implemented a yoga program consisting of 90-minute sessions twice weekly for 12 weeks with 249 perimenopausal women. The results suggested improvements in both sleep quality and mood among the participants,13 yet the study did not include measures of bone density. Although other researchers have explored various forms of mind-body exercises, such as Pilates, baduanjin, and qigong, the findings across these studies could be more consistent.14-17
To date, there has been no comprehensive review evaluating the impact of mind-body exercise interventions on BMD and menopausal symptoms in perimenopausal and postmenopausal women, highlighting the clinical significance of this study.
Methods
The review protocol was registered with PROSPERO on September 11, 2023 (CRD42023459305).
Search strategy
For this study, four electronic databases—PubMed, Embase, Cochrane Central Register of Controlled Trials, and Web of Science—were searched from inception until July 2023. The search strategy used the PICOS framework: (P) Population, perimenopausal and postmenopausal women; (I) Intervention, mind-body exercise; (C) Comparator, control group maintaining daily habits, receiving guidelines to encourage physical activity, and discouraged from other exercise training programs; (O) Outcomes, BMD, sleep quality, anxiety, depression, and fatigue in perimenopausal and postmenopausal women; (S) Study Type, randomized controlled trials (RCT). The detailed search strategy is presented in Table 1 (PubMed is used as an example).
Table 1.
Search strategy on PubMed
| Search | PubMed |
|---|---|
| #1 | Menopause[MeSH Terms] |
| #2 | ((((((Menopause[Title/Abstract]) OR (Perimenopause[Title/Abstract])) OR (Perimenopausal period[Title/Abstract])) OR (Perimenopausal[Title/Abstract])) OR (Postmenopause[Title/Abstract])) OR (postmenopausal period[Title/Abstract])) OR (Postmenopausal[Title/Abstract]) |
| #3 | #1 OR #2 |
| #4 | Tai Ji[MeSH Terms] |
| #5 | Yoga[MeSH Terms] |
| #6 | Qigong[MeSH Terms] |
| #7 | (((((((((((((((((((Mind-body exercise[Title/Abstract]) OR (Mind-body practices[Title/Abstract])) OR (Pilates[Title/Abstract])) OR (Baduanjin[Title/Abstract])) OR (Yoga[Title/Abstract])) OR (Tai Ji[Title/Abstract])) OR (Tai-ji[Title/Abstract])) OR (Tai Chi[Title/Abstract])) OR (Chi, Tai[Title/Abstract])) OR (Tai Ji Quan[Title/Abstract])) OR (Ji Quan, Tai[Title/Abstract])) OR (Quan, Tai Ji[Title/Abstract])) OR (Taiji[Title/Abstract])) OR (Taijiquan[Title/Abstract])) OR (T'ai Chi[Title/Abstract])) OR (Tai Chi Chuan[Title/Abstract])) OR (Qigong[Title/Abstract])) OR (Qi Gong[Title/Abstract])) OR (Ch'i Kung[Title/Abstract])) OR (Chi Kung[Title/Abstract]) |
| #8 | #4 OR #5 OR #6 OR #7 |
| #9 | randomzied controlled trials[Publication Type] |
| #10 | #3 AND #8 AND #9 |
Inclusion criteria
The study included interventions involving mind-body exercises such as tai chi, yoga, Pilates, qigong, baduanjin, and mindfulness-based stress reduction, targeting perimenopausal or postmenopausal groups. The control group received only routine care and rehabilitation or engaged in daily activities without specific interventions. Eligible studies were randomized controlled clinical trials with outcome measures that included at least one of the following: BMD, sleep quality, anxiety, depression, or fatigue.
Exclusion criteria
Excluded were studies with incomplete or unreported data and those originating from non-RCT, including quasi-RCT, animal studies, protocols, conference abstracts, case reports, or correspondence.
Research selection
For research selection, the bibliographic management software EndNote was used. Initially, two researchers independently screened titles for duplicated literature, non-RCT studies, review papers, conference papers, protocols, and communications. Subsequently, abstracts were reviewed to identify relevant studies for inclusion or exclusion. Finally, the remaining literature was thoroughly evaluated by both researchers for final inclusion. In cases of discrepancy, a third researcher was consulted for resolution.
DATA EXTRACTION
Two reviewers independently extracted data using a predefined extraction form, which a third reviewer then validated. A standardized 10-item data extraction form was used to record data for inclusion in the study. The cast included the following headings: (1) author, (2) country, (3) year of publication, (4) population, (5) mean age, (6) total number of participants, (7) details of the exercise intervention, (8) control group, (9) outcomes, and (10) bias domains.
RISK OF BIAS IN INDIVIDUAL STUDIES
Two researchers independently assessed the risk of bias (ROB) in RCT using the Cochrane Handbook version 5.1.0 tool. The assessment considered seven domains: (1) random sequence generation, (2) allocation concealment, (3) blinding of participants and personnel, (4) blinding of outcome assessment, (5) incomplete outcome data, (6) selective reporting, and (7) other biases. Trials were categorized into three levels of ROB based on the number of domains with potentially high risk: high risk (five or more parts), moderate risk (three or four fields), and low risk (two or fewer domains). In cases of missing or unclear information, authors were contacted for clarification.
DATA ANALYSIS
In the included studies where mind-body exercise was used as an intervention, all variables were continuous and expressed as means with standard deviations (SD). Continuous variables were analyzed using the 95% CI and mean difference (MD), where MD is defined as the absolute difference between the means of the intervention and control groups, calculated on the same scale. Alternatively, the standardized mean difference (SMD) was used, representing the MD between groups divided by the standard deviation of participant outcomes, facilitating data consolidation across trials with different scales. A fixed-effect model was applied when I2 was less than 50%; a random-effects model was used for I2 values greater than 50%.
RESULTS
Search results and study design
The initial search strategy yielded 496 records from electronic databases. Reviewers J.L. and P.L. screened the titles and abstracts of 321 references, excluding 247 unrelated records. Subsequently, 74 full-text articles were assessed for eligibility, resulting in the exclusion of 63 articles due to various reasons: 25 were non-RCT, one had incomplete data, nine were conference papers, 13 had results not pertinent to this review, and 15 involved interventions not relevant to this review. Ultimately, 11 studies met the eligibility criteria for inclusion. The research selection process is depicted in Figure 1.
FIG. 1.
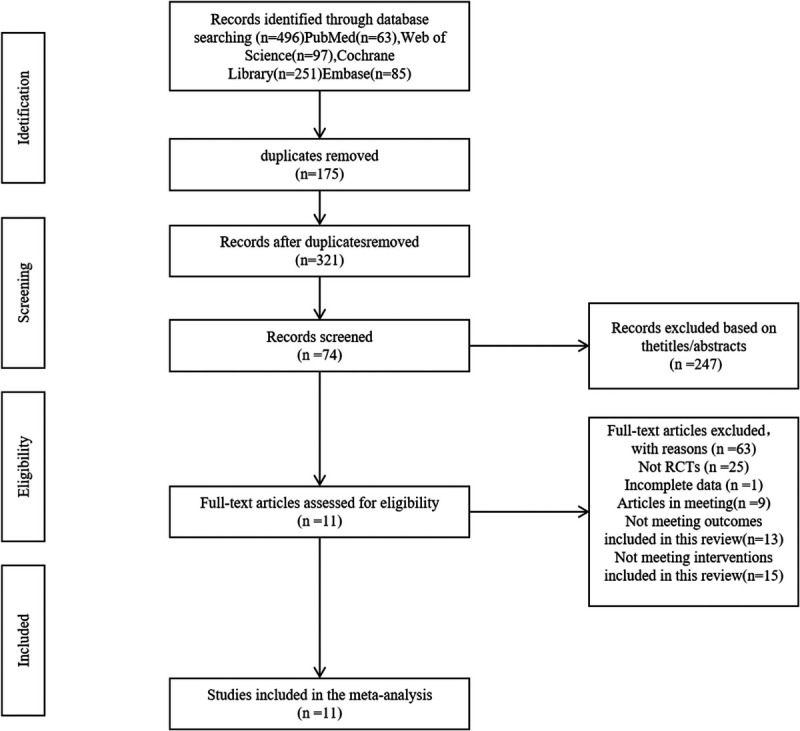
The flowchart of research selection.
Quality assessment of the included studies
There were 11 RCTs involving 1,005 participants (502 perimenopausal and 503 postmenopausal women) from six countries. The studies originated from China (three), Brazil (two), Spain (two), Western Australia (two), Turkey (one), and Canada (one). The included studies explored various mind-body exercises: Pilates (three studies), yoga (three studies), tai chi (three studies), qigong (one study), and mindfulness-based stress reduction (one study). The duration of the interventions ranged from 6 to 48 weeks, with exercise frequencies of 1-3 times per week, each session lasting 1-2.5 hours. Mindfulness meditation studies involved 7 hours of concentrated practice on weekends. The control groups engaged only in routine physical activities, avoiding specific exercise interventions. Outcome measures included BMD, sleep quality, anxiety, depression, and fatigue, assessed using diverse tools. Detailed characteristics of the included studies are provided in Table 2.
Table 2.
Detailed characteristics of the 11 included studies
| Author | Country | Year | Population | Age(mean + SD) | Total | Intervention | Control | Outcome | The Bias Domains |
|---|---|---|---|---|---|---|---|---|---|
| Aibar-Almazán et al19 | Spain | 2019 | Postmenopausal women | T: 69.98 (7.83) C: 66.79 (10.14) |
T: 55 C: 52 |
Pilates Length of intervention: 12 wk Freq: 2 times a week Duration: 1 h |
Con | PSQI FSS HADS |
Random sequence generation (selection bias)(−) Allocation concealment (selection bias)(−) Blinding of participants and personnel (performance bias)(+) Blinding of outcome assessment (detection bias)(−) Incomplete outcome data (attrition bias)(−) Selective reporting (reporting bias)(−) Other bias(−) |
| Buchanan et al21 | Seattle, Washington | 2017 | Perimenopausal women | T: 55.3 (3.9) C: 54.2 (3.7) |
T: 18 C: 17 |
Yoga Length of intervention: 12 wk Freq: 1 time a week Duration: 1.5 h |
Con | PSQI | Random sequence generation (selection bias)(−) Allocation concealment (selection bias)(?) Blinding of participants and personnel (performance bias)(+) Blinding of outcome assessment (detection bias)(−) Incomplete outcome data (attrition bias)(−) Selective reporting (reporting bias)(−) Other bias (−) |
| Angin et al18 | Turkey | 2015 | Postmenopausal women | T: 58.23 (5.46) C: 55.95 (9.22) |
T: 22 C: 19 |
Pilates Length of intervention: 24 wk Freq: 3 times a week Duration: 1 h |
Con | BMD | Random sequence generation (selection bias)(−) Allocation concealment (selection bias)(?) Blinding of participants and personnel (performance bias)(+) Blinding of outcome assessment (detection bias)(−) Incomplete outcome data (attrition bias)(−) Selective reporting (reporting bias)(−) Other bias (−) |
| Wang et al22 | China | 2015 | Postmenopausal women | T: 58.54 (3.37) C: 58.54 (3.37) |
T: 34 C: 35 |
Tai chi Length of intervention: 12 mo Freq: 4 times a week Duration: 1 h |
Con | BMD | Random sequence generation (selection bias)(−) Allocation concealment (selection bias)(−) Blinding of participants and personnel (performance bias)(+) Blinding of outcome assessment (detection bias)(?) Incomplete outcome data (attrition bias)(−) Selective reporting (reporting bias)(?) Other bias (−) |
| Gordon et al25 | Canada | 2021 | Perimenopausal women | T: 48.7 (3.0) C: 48.7 (3.7) |
T: 52 C: 52 |
Mindfulness-based stress Length of intervention: 8 wk Freq: NA Duration: 2.5 h one 7-h weekend intensive silent retreat |
Con | CES-D STAI PSQI |
Random sequence generation (selection bias)(−) Allocation concealment (selection bias)(−) Blinding of participants and personnel (performance bias)(+) Blinding of outcome assessment (detection bias)(?) Incomplete outcome data (attrition bias)(−) Selective reporting (reporting bias)(?) Other bias (−) |
| Newton et al13 | Seattle, Washington | 2014 | Perimenopausal women | T: 54.3 (3.9) C: 54.2 (3.5) |
T: 107 C: 142 |
Yoga Length of intervention: 12 wk Freq: 2 times a week Duration: 90 min |
Con | PSQI ISI PHQ-8 GAD-7 |
Random sequence generation (selection bias)(−) Allocation concealment (selection bias)(−) Blinding of participants and personnel (performance bias)(+) Blinding of outcome assessment (detection bias)(?) Incomplete outcome data (attrition bias)(−) Selective reporting (reporting bias)(?) Other bias (−) |
| de Oliveira et al14 | Brazil | 2019 | Postmenopausal women | T: 55.6 (6.8) C: 54.1 (5.3) |
T: 17 C: 17 |
Pilates Length of intervention: 6 mo Freq: 3 times a week Duration: 1 h |
Con | BMD | Random sequence generation (selection bias)(−) Allocation concealment (selection bias)(−) Blinding of participants and personnel (performance bias)(+) Blinding of outcome assessment (detection bias)(−) Incomplete outcome data (attrition bias)(−) Selective reporting (reporting bias)(−) Other bias (−) |
| Carcelén-Fraile et al24 | Spain | 2022 | Postmenopausal women | T: 69.70 (6.15) C: 69.75 (6.76) |
T: 63 C: 62 |
Qigong Length of intervention: 12 wk Freq: 2 times a week Duration: 1 h |
Con | PSQI HADS |
Random sequence generation (selection bias)(−) Allocation concealment (selection bias)(−) Blinding of participants and personnel (performance bias)(+) Blinding of outcome assessment (detection bias)(−) Incomplete outcome data (attrition bias)(−) Selective reporting (reporting bias)(−) Other bias (−) |
| Afonso et al20 | Brazil | 2012 | Postmenopausal women | T + C: NA | T: 15 C: 15 |
Yoga Length of intervention: 4 mo Freq: 2 times a week Duration: 1 h |
Con | ISI BAI BDI |
Random sequence generation (selection bias)(−) Allocation concealment (selection bias)(−) Blinding of participants and personnel (performance bias)(+) Blinding of outcome assessment (detection bias)(?) Incomplete outcome data (attrition bias)(−) Selective reporting (reporting bias)(?) Other bias (−) |
| Xiao et al23 | China | 2015 | Perimenopausal women | T + C: 55.5 (NA) | T: 20 C: 20 |
Tai chi ball Length of intervention: 6 mo Freq: 3 times a week Duration: 1–2 h |
Con | BMD | Random sequence generation (selection bias)(−) Allocation concealment (selection bias)(−) Blinding of participants and personnel (performance bias)(+) Blinding of outcome assessment (detection bias)(?) Incomplete outcome data (attrition bias)(−) Selective reporting (reporting bias)(?) Other bias (−) |
| Jing15 | China | 2020 | Perimenopausal women | T: 49.7 (3.9) C: 49.7 (4.9) |
T: 36 C: 38 |
Tai chi chuan Length of intervention: 48 wk Freq: 3 times a week Duration: 1 h |
Con | BMD Kupperman scale score |
Random sequence generation (selection bias)(−) Allocation concealment (selection bias)(?) Blinding of participants and personnel (performance bias)(+) Blinding of outcome assessment (detection bias)(?) Incomplete outcome data (attrition bias)(−) Selective reporting (reporting bias)(?) Other bias (−) |
BAI, Beck Anxiety Inventory; BDI, Beck Depression Inventory; BMD, bone mineral density; C, control group; CES-D, Center for Epidemiologic Studies Depression Scale; Con, control group with routine care (no exercise); Freq, frequency; FSS, Fatigue Severity Scale; GAD-7, Generalized Anxiety Disorder questionnaire; HADS, Hospital Anxiety and Depression Scale; ISI, Insomnia Severity Index; NA, unavailable; PHQ-8, Patient Health Questionnaire depression domains; PSQI, Pittsburgh Sleep Quality Index; STAI, Spielberger Trait Anxiety Inventory; T, experimental group; T + C, The ages of the experimental and control groups were not reported separately in the study, only the overall age was reported; (−), Low risk of bias; (?), Unclear risk of bias; (+), High risk of bias.
Risk of bias in the included studies
Figure 2A and B illustrates the ROB assessment for the included studies. All 11 studies used random tables or computerized randomization methods, with 8 reporting adequate allocation concealment. Regarding investigator blinding, 11 studies were identified as partially implementing single-masked procedures, leading to a high risk of performance bias classification. The blinding of participants was described in some studies involving the distribution of envelopes with participant names and group assignments. However, this method was deemed challenging and thus considered a high risk. Five studies explicitly mentioned blinded evaluation of outcome measures. Eleven studies showed consistency with their reported experimental designs, indicating a low risk of reporting bias. Six studies were classified as having “unknown” bias due to unclear baseline characteristics between control and experimental groups. Regarding attrition, 11 trials were considered low risk, as they either provided detailed withdrawal information or conducted an intention-to-treat analysis. Overall, the ROB assessment rated five studies as low risk, five as medium risk, and one as high risk.
FIG. 2.
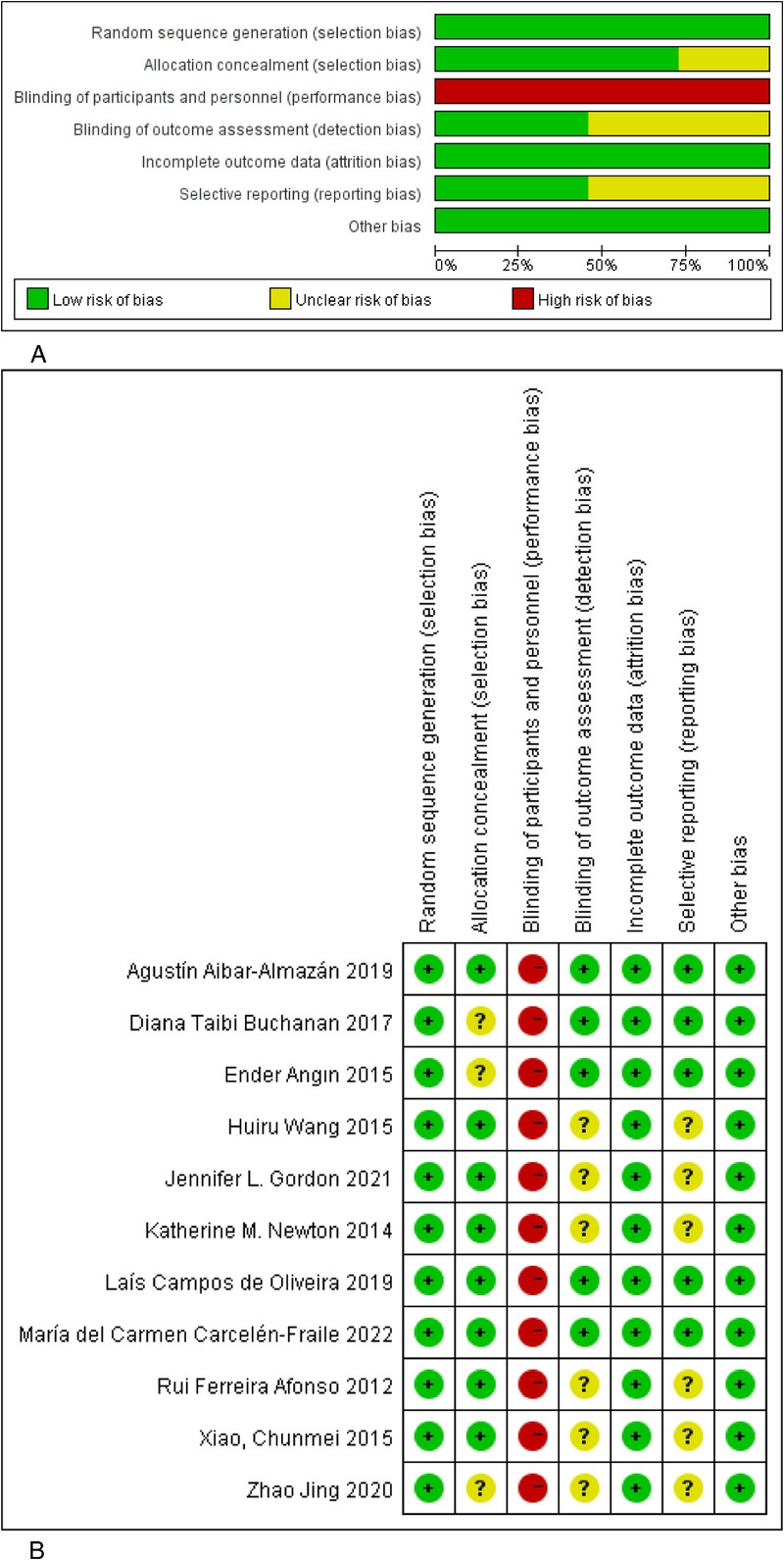
(A) Risk of bias graph for included studies. (B) Risk of bias summary for included studies. (−), Low risk of bias; (?), Unclear risk of bias; (+), High risk of bias.
Effects of mind-body exercise on BMD
Five articles focusing on the impact of mind-body exercise on BMD involved 258 participants. Two studies examined the Pilates exercise's effect on BMD in postmenopausal women. One study, with 41 participants (22 in the Pilates group and 19 in the control group), found a significant increase in BMD values in the Pilates group (P < 0.05). Another, including 34 participants (17 in each group), reported a more substantial improvement in lumbar spine BMD in the Pilates group compared with the control group (P = 0.008).
Three studies assessed tai chi's effect on BMD in postmenopausal women. The first, with 74 participants (36 in the experimental group and 38 in the control group), indicated that 48 weeks of tai chi exercise did not significantly affect the lumbar spine and proximal femur BMD (P > 0.05). However, a decline in BMD was observed in the control group after 48 weeks, suggesting tai chi may mitigate age-related BMD reduction. The second study, including 69 participants (34 in the experimental and 35 in the control group), demonstrated that tai chi could reduce bone loss in postmenopausal women. The third study evaluated the effect of the use of tai chi balls on BMD in perimenopausal women, with 40 participants (20 in each group). It showed significant increases in BMD at L2 (P = 0.048), L3 (P = 0.04), and L4 (P = 0.04), indicating a positive effect.
Heterogeneity testing yielded an I2 of 7%, leading to the selecting of a fixed-effects model for statistical analysis. As Figure 3A illustrates, the SMD was 0.41, with a 95% CI of 0.17 to 0.66 (P = 0.002). This finding indicates that tai chi- and Pilates-based mind-body exercises significantly improve BMD in perimenopausal and postmenopausal women (SMD, 0.41; 95% CI, 0.17 to 0.66; P = 0.001, I2 = 7%).
FIG. 3.
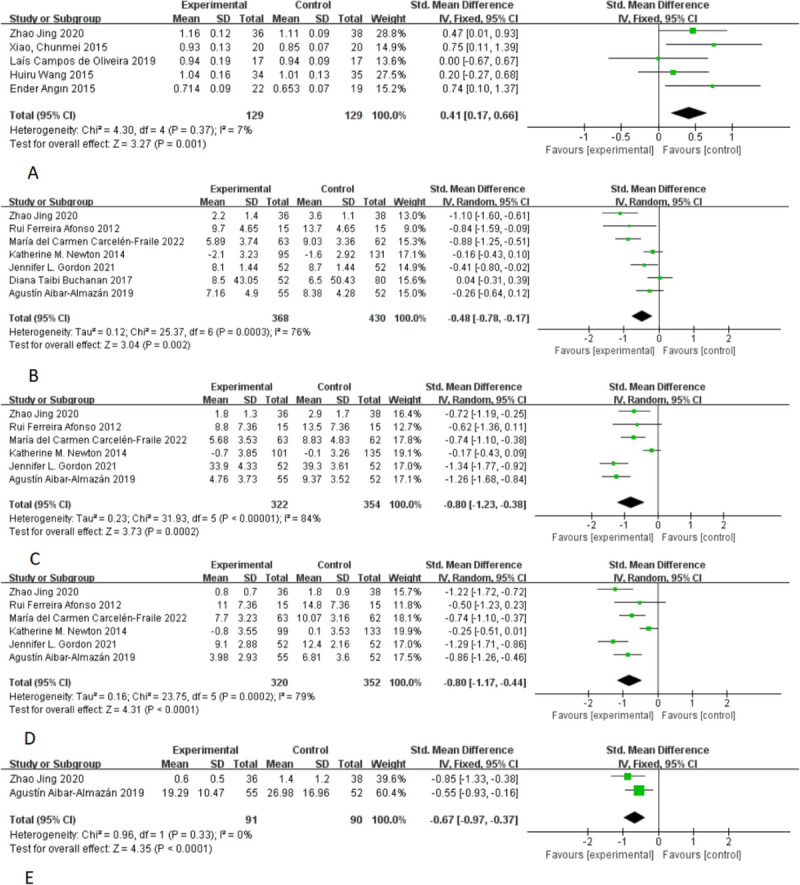
Forest plots show individual and combined effect size estimates and 95% CI for studies on the effects of mind-body exercise interventions. Horizontal lines represent 95% CI, boxes represent study-specific weights, and diamonds represent combined effect sizes. (A) bone mineral density; (B) sleep quality; (C) anxiety; (D) depression; (E) fatigue.
Impact of mind-body exercise on sleep quality
This study analyzed seven articles, encompassing 798 participants, to assess the effect of mind-body exercise on sleep quality. A lower score indicates a more positive impact. The heterogeneity test revealed an I2 value of 76%, leading to the selecting of a random-effects model for statistical analysis. As depicted in Figure 3B, the SMD was −0.48 with a 95% CI of −0.78 to −0.17 (P = 0.002). These results demonstrate that mind-body exercise, compared with control groups, significantly enhances sleep quality in perimenopausal and postmenopausal women.
Effect of mind-body exercise on anxiety
This analysis incorporated six studies with a total of 676 participants to examine the impact of mind-body exercise on anxiety levels. A lower score was indicative of a more favorable outcome. The heterogeneity test yielded an I2 value of 84%, prompting the selection of a random-effects model for the statistical analysis. As presented in Figure 3C, the SMD was −0.80 with a 95% CI of −1.23 to −0.38 (P = 0.0002). These findings indicate that mind-body exercise significantly alleviates anxiety in perimenopausal and postmenopausal women when compared with control groups.
Impact of mind-body exercise on depression
This study analyzed six articles involving 672 participants to evaluate the effect of mind-body exercise on depression. A lower score indicated a more positive outcome. The heterogeneity test revealed an I2 value of 79%, leading to the adopting of a random-effects model for statistical analysis. As shown in Figure 3D, the SMD was −0.80, with a 95% CI of −1.17 to −0.44 (P < 0.0001). These results demonstrate that mind-body exercise considerably reduces depression in perimenopausal and postmenopausal women when compared with control groups.
Effect of mind-body exercise on fatigue
This study reviewed two articles with 181 participants to assess the impact of mind-body exercise on fatigue. A lower score indicated a more favorable effect. The heterogeneity test yielded an I2 of 0%, leading to the application of a fixed-effect model for the statistical analysis. As displayed in Figure 3E, the SMD was −0.67, with a 95% CI of −0.97 to −0.37 (P < 0.0001). These findings suggest that mind-body exercise significantly alleviates fatigue in perimenopausal and postmenopausal women compared with control groups.
PUBLICATION BIAS
Funnel plot analyses for BMD, sleep quality, anxiety, depression, and fatigue were conducted and are depicted in Figure 4A–E. We assessed the symmetry of these funnel plots visually to determine the presence of publication bias. The stories appeared roughly symmetrical on both sides, suggesting an absence of significant publication bias.
FIG. 4.
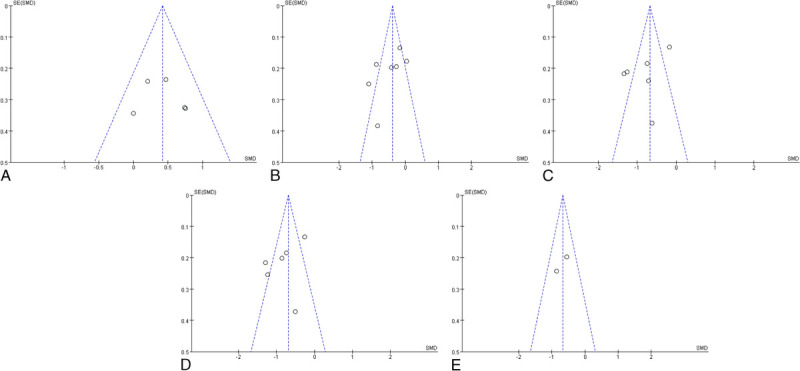
(A) Funnel plot of bone mineral density. (B) Funnel plot of sleep quality. (C) Funnel plot of anxiety. (D) Funnel plot of depression. (E) Funnel plot of fatigue.
SUMMARY OF MAIN RESULTS
This review analyzed the impact of mind-body exercises, compared with no specific exercise intervention (ie, only daily physical activity), on perimenopausal and postmenopausal women across 11 studies. It encompassed five distinct mind-body exercise interventions, namely, Pilates,14,18,19 yoga,13,20,21 tai chi,15,22,23 qigong,24 and mindfulness-based stress reduction,25 involving a total of 1,005 participants, which constitutes a relatively large sample size. Despite variations in exercise frequency and duration across these studies, all the programs included physical movements and regular breathing. The findings indicate that mind-body exercise is a safe and effective intervention, capable of reducing bone loss, enhancing sleep quality, alleviating anxiety and depression, and relieving fatigue in perimenopausal and postmenopausal women.
BONE HEALTH IN PERIMENOPAUSAL AND POSTMENOPAUSAL WOMEN
In perimenopausal and postmenopausal women, the decline in estrogen disrupts the balance between bone formation and absorption, resulting in reduced bone mass and potential deterioration of bone tissue microstructure, thereby increasing the risk of osteoporosis.26 Chan et al27 found that practicing tai chi for 48 weeks at a frequency of five times per week significantly improved the BMD of the trabecular and cortical bone in postmenopausal women. Various meta-analyses support tai chi's effectiveness in slowing bone density loss in this demographic.28,29 This benefit may be attributed to tai chi's focus on abdominal breathing and exercises that target the diaphragm and minor muscle groups around the waist. Prolonged practice changes the pressure exerted by the lower limbs and waist muscles, influencing bone shape intrabone fluid movement, promoting blood circulation within bones, enhancing bone nutrition, and increasing bone mass, thereby affecting bone density.30 Angin et al18 demonstrated that practicing Pilates three times weekly for 6 months significantly increased lumbar bone density, exhibiting a substantial osteogenic effect. Although yoga has been shown to increase bone density, the evidence from randomized controlled studies remains limited.12 The evaluation by Li et al31 of the impact of mind-body exercise on osteoporosis in older adults suggested potential therapeutic effects. Zhang et al32 posited that mind-body practice is optimal for improving BMD in the lumbar spine and femoral neck. This review included five randomized controlled studies, revealing that mind-body exercise (three tai chi sessions and two Pilates sessions) markedly increased bone mineral content and enhanced bone health in perimenopausal and postmenopausal women. These effects may be due to skeletal muscle stress regulating bone mass. Regular and systematic overload exercise improves skeletal muscle cell function, inducing stress on bones, modulating bone metabolism, increasing intraosseous blood volume, facilitating calcium exchange between osteoclasts and osteoblasts along with growth factors, stimulating osteoblast activity, inhibiting bone resorption, and promoting bone formation.33
IMPACT OF MIND-BODY EXERCISE ON SLEEP DISORDERS IN MENOPAUSE
Sleep disorders, affecting 28% to 63% of menopausal women,34 are commonly linked to reduced quality of life and increased comorbidities. Sleep quality, being subjective, is often evaluated using the Pittsburgh Sleep Quality Index (PSQI). Chen et al35 analyzed three RCT with 60 participants, finding that Pilates positively influenced sleep quality. Similarly, Aibar-Almazán et al18 used PSQI to assess sleep in perimenopausal and postmenopausal women, observing significant improvements across all PSQI domains, including the total score, with small- to medium-sized effects. This review also used various tools for sleep assessment, including the Insomnia Severity Index (ISI) in one study, Kupperman's sleep portion in another, and PSQI in the remaining studies. The findings indicate that mind-body exercises such as yoga, Pilates, tai chi, qigong, and mindfulness meditation effectively enhance sleep quality in perimenopausal and postmenopausal women. This is consistent with the systematic reviews by D'Aurea et al36 and Neuendorf et al,37 which suggest that exercise interventions significantly reduce insomnia severity. Potential mechanisms include increased autonomic response to stress, altered sensitivity to chemical and baroreflex responses, enhanced parasympathetic stimulation via the vagus nerve, synchronized cortical regions mediated by the thalamic nucleus, reduced executive function cortical areas, limbic system activation, and increased prolactin and oxytocin secretion.38 Meditation practices activate neural structures involved in attention and autonomic nervous system control,39 facilitating sleep initiation. Yoga practice raises melatonin levels, an essential hormone in sleep regulation, and brain concentrations of gamma-aminobutyric acid. This inhibitory neurotransmitter modulates sympathetic and parasympathetic tones and improves sleep patterns.40,41 Regular exercise promotes brain health by inducing brain-derived neurotrophic factors and mRNA in the hippocampus, which offer neuronutrition and neuroprotection.36,42 In addition, exercise increases energy expenditure, endorphin release, and body temperature, further benefiting sleep.43
MENOPAUSE AND EMOTIONAL DISORDERS
Menopause is linked to an elevated risk of emotional disorders. Research indicates that, compared with men, women experience more adverse life events, self-esteem issues, and reduced quality of life during menopause, significantly impacting the psychological well-being of perimenopausal and postmenopausal women. The most prevalent conditions during this stage are anxiety, depression, and insomnia.44-48 Anxiety and depression often coexist, manifesting as low mood, irritability, nervousness, and, in severe cases, even suicidal tendencies. A large cross-sectional study among Chinese women aged 40-60 years reported that 19.5% and 14.2% of perimenopausal and postmenopausal women, respectively, experienced symptoms of depression and anxiety. This study also found that women engaging in regular physical activity had a 39% and 33% lower risk of developing symptoms of depression and anxiety, respectively, compared with inactive women.49 Our research indicates that mind-body exercises such as yoga, Pilates, tai chi, qigong, and mindfulness meditation can significantly alleviate anxiety, depression, and other negative emotions in perimenopausal and postmenopausal women, corroborating the findings of previous meta-analyses.50,51 The study by Harder et al52 on exercise's genetic susceptibility to depression suggests that physical activity positively influences brain-derived neurotrophic factors, thereby improving mood. Furthermore, exercise has been shown to effectively regulate emotions such as stress and anxiety, enhancing the capacity to manage increased pressure.53
FATIGUE IN PERIMENOPAUSAL AND POSTMENOPAUSAL WOMEN
Fatigue is a prevalent concern among perimenopausal and postmenopausal women and is a common reason for medical consultations in this demographic. The literature on the efficacy of physical exercise in mitigating fatigue symptoms is mixed. At the same time, some studies suggest that physical activity can alleviate fatigue and its associated symptoms, but others have not established a clear link between exercise and fatigue reduction.54,55 Recent meta-analyses incorporating several controlled trials with inactive control groups have reported inconsistent findings due to small sample sizes and variable study quality. However, these analyses support the notion that Pilates can help alleviate fatigue in older adults.56 In our study, postmenopausal women engaging in mind-body exercises (tai chi, Pilates) demonstrated significantly lower scores on the Kupperman fatigue component and the Fatigue Severity Scale (FSS) than those in the daily activity group. However, as only two studies were included in this analysis, the conclusion that mind-body exercise alleviates fatigue in perimenopausal and postmenopausal women should be cautiously approached.
STRENGTHS AND LIMITATIONS
According to Kontis et al,57 future life expectancy projections for women in 35 industrialized countries indicate a 50% chance of surpassing 90 years by 2030. With an aging population, the management of perimenopausal and postmenopausal women is increasingly emphasized.58 Hormone replacement therapy, commonly used to alleviate menopausal symptoms,59,60 has been linked to potential cancer risks.61-63 Recently, mind-body exercises have emerged as innovative approaches to improve menopausal symptoms and bone health. However, the research in this area presents inconsistent findings, highlighting the importance of this systematic review of mind-body exercise's effects on menopause. Our study encompassed 1,005 perimenopausal and postmenopausal women, representing a substantial sample size, and exclusively included RCT. It was conducted by two experienced researchers who meticulously extracted and analyzed data, adhering to the PROSPERO guidelines and prospective registration, ensuring the study's rigor.
Nonetheless, this study also faces limitations:
Some studies did not fully report randomized and blinding methods, raising concerns about measurement and implementation biases.
Due to the nature of the interventions, participants could not be blinded, which might introduce self-reported biases despite unaware of the study hypothesis.
Variations in participants' ages, individual differences, and proficiency in physical and mental exercises could impact the study outcomes.
Inconsistencies in the process, duration, and frequency of mind-body exercises across studies may contribute to clinical heterogeneity and affect outcomes.
Most studies assessing menopausal sleep disorders relied on subjective questionnaires such as the PSQI and ISI, lacking objective sleep indicators. Polysomnography, an accurate tool that records sleep duration and disruptions,64 could provide more reliable sleep quality assessments and is recommended for future research.
CONCLUSIONS
This meta-analysis of RCT evaluates the efficacy of mind-body exercise interventions in enhancing BMD and alleviating menopausal symptoms in perimenopausal and postmenopausal women. The findings indicate that mind-body exercises positively impact BMD, sleep quality, anxiety, depression, and fatigue among this demographic. Consequently, physicians can recommend mind-body practices as an effective nonpharmacological treatment for managing menopausal symptoms. In addition, this approach provides perimenopausal and postmenopausal women with more exercise options. The insights gained from this systematic analysis can be valuable for clinical research and treating menopausal symptoms.
Footnotes
Funding/support: None reported.
Financial disclosure/conflicts of interest: None reported.
Author contributions: H.X. and Y.L. conceived and designed the study; J.L. and P.L. performed the literature search and collected the data; H.X. and J.L. contributed the data analysis and wrote the manuscript; P.L. and Y.L. revised the manuscript. All authors reviewed and approved the manuscript before submission.
Contributor Information
Jian Liu, Email: 280373902@qq.com.
Peishan Li, Email: 53440515@qq.com.
Yujie Liang, Email: 364150666@qq.com.
REFERENCES
- 1.Deng Y, Huang H, Shi J, Jin H. Identification of candidate genes in breast cancer induced by estrogen plus progestogens using bioinformatic analysis. Int J Mol Sci 2022;23:11892. doi: 10.3390/ijms231911892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu T, Yue R, He M, Xu C. Effect of fenugreek on vasomotor symptoms in menopausal women: a protocol for systematic review and meta-analysis. Medicine (Baltimore) 2020;99:e20526. doi: 10.1097/MD.0000000000020526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang M, Wen S, Zhang J, Peng J, Shen X, Xu L. Systematic review and meta-analysis: changes of gut microbiota before and after menopause. Dis Markers 2022;2022:3767373. doi: 10.1155/2022/3767373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zangirolami-Raimundo J Sorpreso ICE Rebouças CMP, et al. Depression in women in climacteric period: a brief review. Rev Assoc Med Bras (1992) 2023;69:e20230385. doi: 10.1590/1806-9282.20230385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costa-Hong VA, Muela HCS, Macedo TA, Sales ARK, Bortolotto LA. Gender differences of aortic wave reflection and influence of menopause on central blood pressure in patients with arterial hypertension. BMC Cardiovasc Disord 2018;18:123. doi: 10.1186/s12872-018-0855-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monteleone P, Mascagni G, Giannini A, Genazzani AR, Simoncini T. Symptoms of menopause—global prevalence, physiology and implications. Nat Rev Endocrinol 2018;14:199–215. doi: 10.1038/nrendo.2017.180 [DOI] [PubMed] [Google Scholar]
- 7.Capel-Alcaraz AM, García-López H, Castro-Sánchez AM, Fernández-Sánchez M, Lara-Palomo IC. The efficacy of strength exercises for reducing the symptoms of menopause: a systematic review. J Clin Med 2023;12:548. doi: 10.3390/jcm12020548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park E, Lim E, Yeo S, Yong Y, Yang J, Jeong SY. Anti-menopausal effects of cornus officinalis and ribes fasciculatum extract in vitro and in vivo. Nutrients 2020;12:369. doi: 10.3390/nu12020369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas A, Daley AJ. Women's views about physical activity as a treatment for vasomotor menopausal symptoms: a qualitative study. BMC Womens Health 2020;20:203. doi: 10.1186/s12905-020-01063-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehrnoush V, Darsareh F, Roozbeh N, Ziraeie A. Efficacy of the complementary and alternative therapies for the management of psychological symptoms of menopause: a systematic review of randomized controlled trials. J Menopausal Med 2021;27:115–131. doi: 10.6118/jmm.21022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Innes KE, Selfe TK, Vishnu A. Mind-body therapies for menopausal symptoms: a systematic review. Maturitas 2010;66:135–149. doi: 10.1016/j.maturitas.2010.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Motorwala ZS, Kolke S, Panchal PY, Bedekar NS, Sancheti PK, Shyam A. Effects of yogasanas on osteoporosis in postmenopausal women. Int J Yoga 2016;9:44–48. doi: 10.4103/0973-6131.171717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newton KM Reed SD Guthrie KA, et al. Efficacy of yoga for vasomotor symptoms: a randomized controlled trial. Menopause 2014;21:339–346. doi: 10.1097/GME.0b013e31829e4baa [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Oliveira LC, de Oliveira RG, de Almeida Pires-Oliveira DA. Effects of whole-body vibration versus Pilates exercise on bone mineral density in postmenopausal women: a randomized and controlled clinical trial. J Geriatr Phys Ther 2019;42:E23–E31. doi: 10.1519/JPT.0000000000000184 [DOI] [PubMed] [Google Scholar]
- 15.Jing Z. Effects of tai chi chuan on the changes of bone mineral density of perimenopausal women. Chin J Tissue Eng Res 2020;24:176–180. doi: 10.3969/j.issn.2095-4344.1908 [DOI] [Google Scholar]
- 16.Shorey S, Ang L, Lau Y. Efficacy of mind-body therapies and exercise-based interventions on menopausal-related outcomes among Asian perimenopause women: a systematic review, meta-analysis, and synthesis without a meta-analysis. J Adv Nurs 2020;76:1098–1110. doi: 10.1111/jan.14304 [DOI] [PubMed] [Google Scholar]
- 17.Sun C Qi B Huang X, et al. Baduanjin exercise: a potential promising therapy toward osteoporosis. Front Med (Lausanne) 2022;9:935961. doi: 10.3389/fmed.2022.935961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Angin E, Erden Z, Can F. The effects of clinical Pilates exercises on bone mineral density, physical performance and quality of life of women with postmenopausal osteoporosis. J Back Musculoskelet Rehabil 2015;28:849–858. doi: 10.3233/BMR-150604 [DOI] [PubMed] [Google Scholar]
- 19.Aibar-Almazán A, Hita-Contreras F, Cruz-Díaz D, de la Torre-Cruz M, Jiménez-García JD, Martínez-Amat A. Effects of Pilates training on sleep quality, anxiety, depression and fatigue in postmenopausal women: a randomized controlled trial. Maturitas 2019;124:62–67. doi: 10.1016/j.maturitas.2019.03.019 [DOI] [PubMed] [Google Scholar]
- 20.Afonso RF Hachul H Kozasa EH, et al. Yoga decreases insomnia in postmenopausal women: a randomized clinical trial. Menopause 2012;19:186–193. doi: 10.1097/gme.0b013e318228225f [DOI] [PubMed] [Google Scholar]
- 21.Buchanan DT Landis CA Hohensee C, et al. Effects of yoga and aerobic exercise on actigraphic sleep parameters in menopausal women with hot flashes. J Clin Sleep Med 2017;13:11–18. doi: 10.5664/jcsm.6376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H, Yu B, Chen W, Lu Y, Yu D. Simplified tai chi resistance training versus traditional tai chi in slowing bone loss in postmenopausal women. Evid Based Complement Alternat Med 2015;2015:379451. doi: 10.1155/2015/379451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiao C, Kang Y, Zhuang YC. Effects of tai chi ball on estrogen levels, bone metabolism index, and muscle strength of perimenopausal women. J Am Geriatr Soc 2015;63:2629–2631. doi: 10.1111/jgs.13862 [DOI] [PubMed] [Google Scholar]
- 24.Carcelén-Fraile MDC Aibar-Almazán A Martínez-Amat A, et al. Qigong for mental health and sleep quality in postmenopausal women: a randomized controlled trial. Medicine (Baltimore) 2022;101:e30897. doi: 10.1097/MD.0000000000030897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gordon JL, Halleran M, Beshai S, Eisenlohr-Moul TA, Frederick J, Campbell TS. Endocrine and psychosocial moderators of mindfulness-based stress reduction for the prevention of perimenopausal depressive symptoms: a randomized controlled trial. Psychoneuroendocrinology 2021;130:105277. doi: 10.1016/j.psyneuen.2021.105277 [DOI] [PubMed] [Google Scholar]
- 26.Mirkin S, Pickar JH. Management of osteoporosis and menopausal symptoms: focus on bazedoxifene/conjugated estrogen combination. Int J Womens Health 2013;5:465–475. doi: 10.2147/IJWH.S39455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan K Qin L Lau M, et al. A randomized, prospective study of the effects of tai chi chun exercise on bone mineral density in postmenopausal women. Arch Phys Med Rehabil 2004;85:717–722. doi: 10.1016/j.apmr.2003.08.091 [DOI] [PubMed] [Google Scholar]
- 28.Wu HY Wang YR Wen GW, et al. Tai chi on bone mineral density of postmenopausal osteoporosis: a protocol for systematic review and meta-analysis. Medicine (Baltimore) 2020;99:e21928. doi: 10.1097/MD.0000000000021928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu X Jiang C Fan R, et al. The effect and safety of tai chi on bone health in postmenopausal women: a meta-analysis and trial sequential analysis. Front Aging Neurosci 2022;14:935326. doi: 10.3389/fnagi.2022.935326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song QH Zhang QH Xu RM, et al. Effect of Tai-chi exercise on lower limb muscle strength, bone mineral density and balance function of elderly women. Int J Clin Exp Med 2014;7:1569–1576. PMID: 25035781 [PMC free article] [PubMed] [Google Scholar]
- 31.Li H Jiang H Wang J, et al. Effects of mind-body exercises for osteoporosis in older adults: a systematic review and meta-analysis of randomized controlled trials. Geriatr Orthop Surg Rehabil 2023;14:21514593231195237. doi: 10.1177/21514593231195237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang S Huang X Zhao X, et al. Effect of exercise on bone mineral density among patients with osteoporosis and osteopenia: a systematic review and network meta-analysis. J Clin Nurs 2022;31(15-16):2100–2111. doi: 10.1111/jocn.16101 [DOI] [PubMed] [Google Scholar]
- 33.Fernández-Rodríguez R, Alvarez-Bueno C, Reina-Gutiérrez S, Torres-Costoso A, Nuñez de Arenas-Arroyo S, Martínez-Vizcaíno V. Effectiveness of Pilates and yoga to improve bone density in adult women: a systematic review and meta-analysis. PloS One 2021;16:e0251391. doi: 10.1371/journal.pone.0251391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yazdi Z, Sadeghniiat-Haghighi K, Ziaee A, Elmizadeh K, Ziaeeha M. Influence of sleep disturbances on quality of life of Iranian menopausal women. Psychiatry J 2013;2013:907068. doi: 10.1155/2013/907068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen Z Ye X Shen Z, et al. Effect of Pilates on sleep quality: a systematic review and meta-analysis of randomized controlled trials. Front Neurol 2020;11:158. doi: 10.3389/fneur.2020.00158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.D'Aurea CVR, Frange C, Poyares D, Souza AAL, Lenza M. Physical exercise as a therapeutic approach for adults with insomnia: systematic review and meta-analysis. Einstein (Sao Paulo) 2022;20:eAO8058. doi: 10.31744/einstein_journal/2022AO8058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neuendorf R, Wahbeh H, Chamine I, Yu J, Hutchison K, Oken BS. The effects of mind-body interventions on sleep quality: a systematic review. Evid Based Complement Alternat Med 2015;2015:902708. doi: 10.1155/2015/902708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown RP, Gerbarg PL. Sudarshan Kriya yogic breathing in the treatment of stress, anxiety, and depression: part I-neurophysiologic model. J Altern Complement Med 2005;11:189–201. doi: 10.1089/acm.2005.11.189 [DOI] [PubMed] [Google Scholar]
- 39.Lazar SW, Bush G, Gollub RL, Fricchione GL, Khalsa G, Benson H. Functional brain mapping of the relaxation response and meditation. Neuroreport 2000;11:1581–1585 [PubMed] [Google Scholar]
- 40.Harinath K Malhotra AS Pal K, et al. Effects of Hatha yoga and Omkar meditation on cardiorespiratory performance, psychologic profile, and melatonin secretion. J Altern Complement Med 2004;10:261–268. doi: 10.1089/107555304323062257 [DOI] [PubMed] [Google Scholar]
- 41.Douglass L. Yoga as an intervention in the treatment of eating disorders: does it help? Eat Disord 2009;17:126–139. doi: 10.1080/10640260802714555 [DOI] [PubMed] [Google Scholar]
- 42.Chalimoniuk M, Chrapusta SJ, Lukačova N, Langfort J. Endurance training upregulates the nitric oxide/soluble guanylyl cyclase/cyclic guanosine 3',5'-monophosphate pathway in the striatum, midbrain and cerebellum of male rats. Brain Res 2015;1618:29–40. doi: 10.1016/j.brainres.2015.05.020 [DOI] [PubMed] [Google Scholar]
- 43.Yang PY, Ho KH, Chen HC, Chien MY. Exercise training improves sleep quality in middle-aged and older adults with sleep problems: a systematic review. J Physiother 2012;58:157–163. doi: 10.1016/S1836-9553(12)70106-6 [DOI] [PubMed] [Google Scholar]
- 44.Hachul H Castro LS Bezerra AG, et al. Hot flashes, insomnia, and the reproductive stages: a cross-sectional observation of women from the EPISONO study. J Clin Sleep Med 2021;17:2257–2267. doi: 10.5664/jcsm.9432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Joffe H de Wit A Coborn J, et al. Impact of estradiol variability and progesterone on mood in perimenopausal women with depressive symptoms. J Clin Endocrinol Metab 2020;105:e642–e650. doi: 10.1210/clinem/dgz181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carcelén-Fraile MDC Aibar-Almazán A Martínez-Amat A, et al. Effects of physical exercise on sexual function and quality of sexual life related to menopausal symptoms in peri- and postmenopausal women: a systematic review. Int J Environ Res Public Health 2020;17:2680. doi: 10.3390/ijerph17082680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simbar M, Nazarpour S, Alavi Majd H, Dodel Andarvar K, Jafari Torkamani Z, Alsadat Rahnemaei F. Is body image a predictor of women's depression and anxiety in postmenopausal women? BMC Psychiatry 2020;20:202. doi: 10.1186/s12888-020-02617-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Monfaredi Z, Malakouti J, Farvareshi M, Mirghafourvand M. Effect of acceptance and commitment therapy on mood, sleep quality and quality of life in menopausal women: a randomized controlled trial. BMC Psychiatry 2022;22:108. doi: 10.1186/s12888-022-03768-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang X, Zhao G, Di J, Wang L, Zhang X. Prevalence and risk factors for depressive and anxiety symptoms in middle-aged Chinese women: a community-based cross-sectional study. BMC Womens Health 2022;22:319. doi: 10.1186/s12905-022-01908-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Z, Liu S, Wang L, Smith L. Mind-body exercise for anxiety and depression in COPD patients: a systematic review and meta-analysis. Int J Environ Res Public Health 2019;17:22. doi: 10.3390/ijerph17010022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Villaverde Gutiérrez C Torres Luque G Ábalos Medina GM, et al. Influence of exercise on mood in postmenopausal women. J Clin Nurs 2012;21:923–928. doi: 10.1111/j.1365-2702.2011.03972.x [DOI] [PubMed] [Google Scholar]
- 52.Harder JA Fichorova RN Srivastava A, et al. Brain-derived neurotrophic factor and mood in perimenopausal depression. J Affect Disord 2022;300:145–149. doi: 10.1016/j.jad.2021.12.092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu F, Zhang J, Yang H, Jiang J. The effect of physical exercise on the elderly's anxiety: based on systematic reviews and meta-analysis. Comput Math Methods Med 2022;2022:4848290. doi: 10.1155/2022/4848290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Puetz TW, O'Connor PJ, Dishman RK. Effects of chronic exercise on feelings of energy and fatigue: a quantitative synthesis. Psychol Bull 2006;132:866–876. doi: 10.1037/0033-2909.132.6.866 [DOI] [PubMed] [Google Scholar]
- 55.Elavsky S, Gold CH. Depressed mood but not fatigue mediate the relationship between physical activity and perceived stress in middle-aged women. Maturitas 2009;64:235–240. doi: 10.1016/j.maturitas.2009.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fleming KM, Herring MP. The effects of Pilates on mental health outcomes: a meta-analysis of controlled trials. Complement Ther Med 2018;37:80–95. doi: 10.1016/j.ctim.2018.02.003 [DOI] [PubMed] [Google Scholar]
- 57.Kontis V, Bennett JE, Mathers CD, Li G, Foreman K, Ezzati M. Future life expectancy in 35 industrialised countries: projections with a Bayesian model ensemble. Lancet 2017;389:1323–1335. doi: 10.1016/S0140-6736(16)32381-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Keye C, Varley J, Patton D. The impact of menopause education on quality of life among menopausal women: a systematic review with meta-analysis. Climacteric 2023;26:419–427. doi: 10.1080/13697137.2023.2226318 [DOI] [PubMed] [Google Scholar]
- 59.Meziou N, Scholfield C, Taylor CA, Armstrong HL. Hormone therapy for sexual function in perimenopausal and postmenopausal women: a systematic review and meta-analysis update. Menopause 2023;30:659–671. doi: 10.1097/GME.0000000000002185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pan Z, Wen S, Qiao X, Yang M, Shen X, Xu L. Different regimens of menopausal hormone therapy for improving sleep quality: a systematic review and meta-analysis. Menopause 2022;29:627–635. doi: 10.1097/GME.0000000000001945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lallas K Anagnostis P Theocharis P, et al. The effect of menopausal hormone therapy on the risk of melanoma and keratinocyte skin cancer: a systematic review and meta-analysis of observational studies. Maturitas 2023;168:20–28. doi: 10.1016/j.maturitas.2022.10.010 [DOI] [PubMed] [Google Scholar]
- 62.Chlebowski RT, Aragaki AK. The Women's Health Initiative randomized trials of menopausal hormone therapy and breast cancer: findings in context. Menopause 2023;30:454–461. doi: 10.1097/GME.0000000000002154 [DOI] [PubMed] [Google Scholar]
- 63.Liu JY, Chen TJ, Hwang SJ. The risk of breast cancer in women using menopausal hormone replacement therapy in Taiwan. Int J Environ Res Public Health 2016;13:482. doi: 10.3390/ijerph13050482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tsai SY, Lee PL, Gordon C, Cayanan E, Lee CN. Objective sleep efficiency but not subjective sleep quality is associated with longitudinal risk of depression in pregnant women: a prospective observational cohort study. Int J Nurs Stud 2021;120:103966. doi: 10.1016/j.ijnurstu.2021.103966 [DOI] [PubMed] [Google Scholar]


