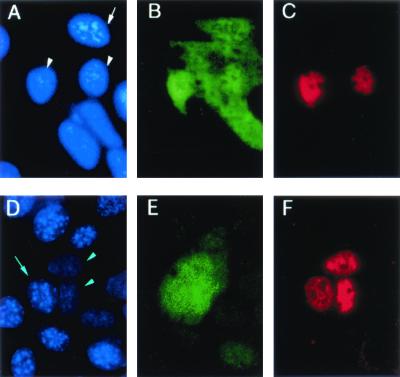
FIG. 11.
Effect of mutant RBP1 on PTB nuclear export in a heterokaryon fusion assay. Human HuH-7 cells were cotransfected with plasmids that express mutant RBP1 fused to GFP and human PTB tagged with a myc epitope. After 2 days, the cells were cocultured with murine NIH 3T3 cells in the presence of cycloheximide and fused for 15 min. RBP1 was localized by the green fluorescence of GFP (B), PTB was localized to the myc epitope by a monoclonal antibody and Cy3-labeled secondary antibody (C), while nuclei were stained with DAPI (A). Two transfected human nuclei (A, arrowheads) can be identified by the presence of red staining for PTB. As expected, mutant RBP1 was present in both the cytoplasm and these two human nuclei. In addition, however, mutant RBP1 was imported into a murine nucleus (A, arrow), indicating that a human-mouse heterokaryon had formed. This murine nucleus remained negative for myc-tagged PTB, whose export from the human nuclei thus must have been blocked by mutant RBP1. A negative control experiment using unfused GFP (E) confirmed that GFP itself has no effect on the export of PTB (F) from human nuclei (D, arrowheads) into murine nuclei (D, arrow).