Abstract
Antiphospholipid antibody syndrome, also known as Hughes syndrome, is a multi-system autoimmune disorder characterized by recurrent thrombosis and fetal loss. The most common types of antiphospholipid antibodies include lupus anticoagulants, anticardiolipin antibodies, and anti-beta 2 glycoprotein 1 antibodies. Owing to the prothrombotic nature of this disease, diagnosing and treating the condition as early as possible is necessary. In this case report, we discuss the typical and atypical manifestations of this disease, as well as the current diagnostic and therapeutic options. We present a case of atypical antiphospholipid antibody syndrome in the form of hemi-tongue ischemia.
Keywords: Antiphospholipid antibody syndrome, Hemitongue ischemia
1. Introduction
Antiphospholipid antibody syndrome (APS) is an autoimmune disorder characterized by hypercoagulability, which leads to arterial and venous thrombosis and obstetric complications. Antiphospholipid antibody syndrome is estimated to affect about 1 in 2000 people, with 20% of those under 50 years of age who have had a stroke developing the condition. Research suggests that 20–30% of patients with systemic lupus erythematosus may have antiphospholipid antibody syndrome, as well as 10–15% of women with pregnancy complications. It is worth noting that the majority of those diagnosed with the syndrome are women, with approximately 70% of cases occurring in this demographic.1 The most commonly implicated antibodies include anti-beta 2 glycoprotein 1, lupus anticoagulant, and anti-cardiolipin. 2 Antiphospholipid antibodies are directed against phospholipids and trigger a cascade involving complements, platelets, endothelial cells, and adhesion molecules, leading to a prothrombotic state.3 The syndrome can either be primary or secondary. The primary APS occurs in the absence of other related diseases. In contrast, secondary APS can be caused by other autoimmune disorders - such as systemic lupus erythematosus. In rare cases, APS can lead to rapid organ failure due to generalized thrombosis, termed catastrophic antiphospholipid syndrome (CAPS), and is associated with high morbidity and mortality.
We present the case of a 63-year-old male who presented to the hospital with tongue pain and discoloration, was diagnosed with hemi-tongue ischemia, and was later with APS.
2. Case presentation
A 63-year-old man presented to our hospital with acute-onset left-sided tongue pain, paresthesia, left-sided jaw pain, and headache. The patient denied experiencing any episodes of pain previously. His medical history included well-controlled hypertension, current active daily smoking, and testicular cancer status after chemotherapy and orchiectomy performed, 30 years ago. Patient reported no pertinent family history.
Upon arrival at the emergency department, his heart rate was 90 beats/min, respiratory rate was 18 breaths/min, oxygen saturation was 98% on room air, and blood pressure was 110/70 mmHg.
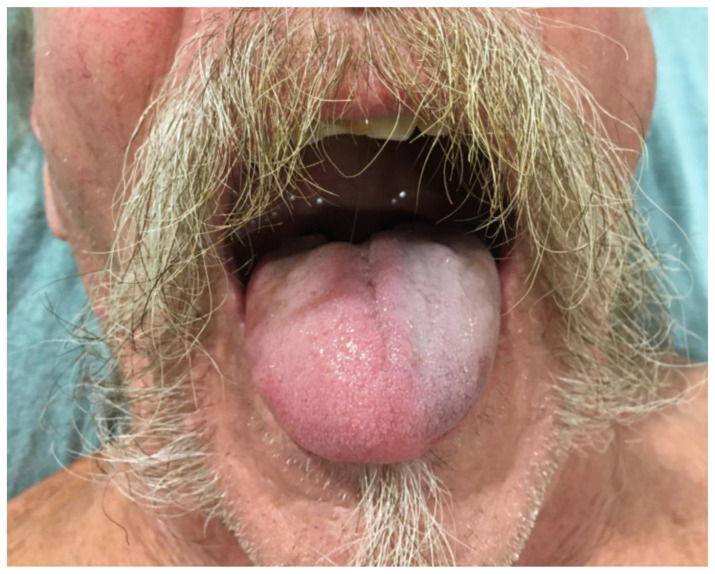
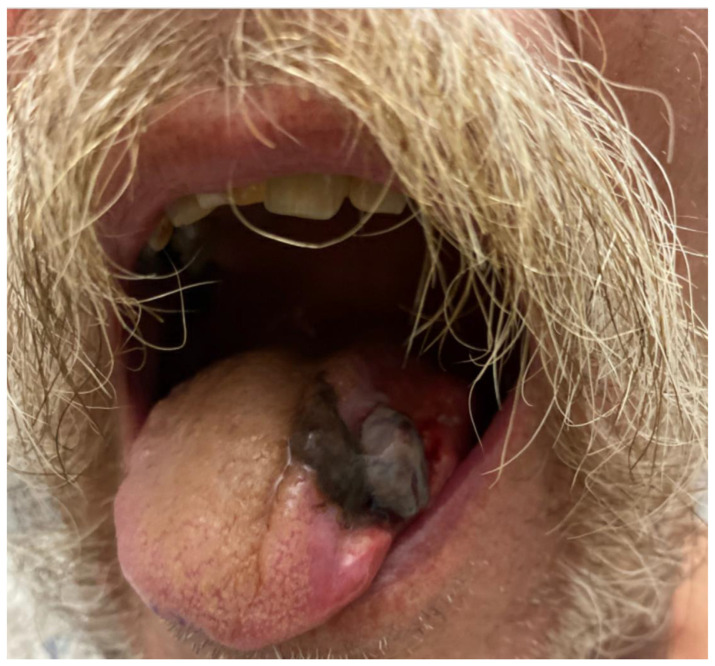
Physical examination was significant for left tongue discoloration (Figs. 1 and 2).
Fig. 1.
Left sided tongue discoloration.
Fig. 2.
Left side of the tongue with necrotic part sloughing off.
Laboratory results, including complete blood count, comprehensive metabolic panel, PT/INR, aPTT, fibrinogen and D-dimer were unremarkable. Inflammatory markers, including C-reactive protein and erythrocyte sedimentation rate, were within normal limits. Computed tomographic angiography of the head and neck revealed, no vessel dissection, occlusion, or stenosis.
Possible differential diagnoses based on reported symptoms and clinical presentation included systemic vasculitis, such as giant cell arteritis (GCA) and anti-neutrophil cytoplasmic antibody associated vasculitis; drug-mediated; thromboembolic and cardioembolic diseases; and connective tissue diseases.
Owing to concerns about thromboembolic disease, the patient was administered intravenous heparin. To rule out a potential cardiac etiology, the patient underwent transthoracic echocardiography, which was negative for acute abnormalities. Cardiology was consulted, and transesophageal echocardiography was performed, which did not reveal any shunt or patent foramen ovale.
Extensive laboratory workup was performed, including anti-nuclear antibody, anti-double-stranded deoxyribonucleic acid, antimyeloperoxidase antibody (pANCA), and anti-proteinase 3 (cANCA). Viral panels, including those for cytomegalovirus, varicella zoster virus, and human immunodeficiency virus/hepatitis C virus, were also sent, and the results were negative. Antiphospholipid antibody panel: beta-2 glycoprotein, lupus anticoagulant, and anticardiolipin antibodies were also sent for testing.
Given the patient’s age and presenting symptoms, including headache, giant cell arteritis was considered one of the top differential diagnoses. A rheumatological analysis was also performed.
The patient was started on high-dose intravenous steroid therapy. Ophthalmology was consulted due to concerns regarding ocular involvement with the GCA, and the patient underwent a bilateral temporal artery biopsy. However, the temporal artery biopsy results were unremarkable.
Four days after hospitalization, antiphospholipid antibody titers were measured, and the patient had elevated beta-2 glycoprotein immunoglobin (Ig) G and anticardiolipin antibody IgM levels. PTT Lupus anticoagulant levels were normal.
The hematology-oncology department was consulted, and warfarin treatment was administered for possible APS. The patient’s symptoms improved during the hospitalization, whereas the necrotic part of the tongue was sloughed.
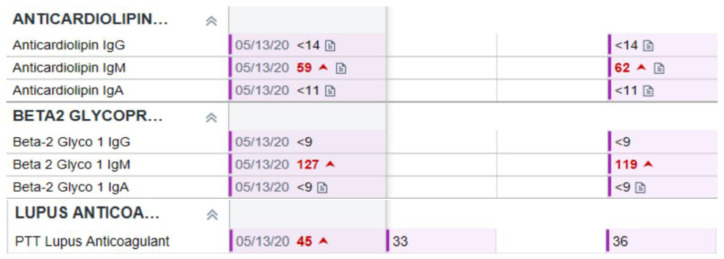
The patient was discharged with close rheumatology and hematology-oncology follow-ups. The patient’s 12 weeks’ antiphospholipid antibody titers, including beta-2 glycoprotein (127 SMU, reference range is < 20 SMU) and anticardiolipin antibodies (59 MPL, reference range is < 20 MPL), were elevated again at follow-up. PTT Lupus anticoagulant levels were elevated at 12 weeks (45 s, reference range is < 40sec) but patient was already on warfarin (Fig. 3).
Fig. 3.
Antibody titers twelve weeks apart.
3. Literature review
We performed a brief literature review of PubMed by searching keywords “Antiphospholipid Antibody Syndrome” AND “Hemi tongue Ischemia/Tongue Ischemia/Lingual thrombosis” and found only one case of hemi-tongue ischemia in a previously diagnosed patient with antiphospholipid antibody syndrome, published in an obstetrics/gynecology journal.
4. Discussion
Antiphospholipid antibody syndrome has many clinical manifestations including recurrent thromboses, repeated miscarriages, cardiac valve vegetation, and thrombocytopenia. It can involve any vascular bed in the body and hence, may present as pulmonary embolism, pulmonary hypertension, stroke, bowel infarction, or renovascular hypertension; in this case, tongue ischemia. Fetal loss seems to stem from antibody-mediated interference with trophoblast growth and differentiation, causing failure of placentation.
Antiphospholipid antibody syndrome originates from the detection of circulating antibodies that bind phospholipids. Most pathological effects are believed to be mediated by the binding of antibodies to epitopes on proteins that are somehow induced or “unveiled” by phospholipids. Antibody targets include B2-glycoprotein 1, a plasma protein associated with the surface of endothelial cells and trophoblasts, and prothrombin. It is suspected that these antibodies bind to these and other proteins to induce a hypercoagulable state via unknown mechanisms.14
We observed isolated tongue ischemia in a pregnant patient previously diagnosed with antiphospholipid antibody syndrome.4 Our clinical case indicates that, in any presentation of tongue ischemia, antiphospholipid antibody syndrome should be considered as a differential diagnosis and should be thoroughly evaluated, as this condition is associated with high morbidity and mortality, and prompt treatment is essential. The updated international consensus (Sydney) classification (ICS) criteria for definite antiphospholipid syndrome require the presence of lupus anticoagulant (LA) and/or IgG or IgM anticardiolipin antibody (aCL) present in medium or high titers (i.e., >40 GPL or MPL or >99th percentile), and/or anti-beta 2 glycoprotein-1 (aβ2GPI) (IgG and/or IgM) > 99th percentile.11 These antiphospholipid antibodies should be persistent and defined as present on two or more consecutive occasions at least 12 weeks apart.11 Laboratory examination revealed elevated levels of anti-beta 2 glycoprotein 1, anti-cardiolipin IgM, and lupus antibodies. The patient was treated with warfarin, and after treatment, he was discharged with rheumatology and hematology follow-up. Multiple case reports have shown that tongue infarction is the initial presenting symptom of giant cell arteritis/temporal arteritis.5 There have been case reports of tongue necrosis due to disseminated intravascular coagulation (DIC),6 carotid artery stenosis,7 rheumatoid hyperviscosity,8 cardiogenic shock,9 and use of vasoconstricting agents in the ICU.10
Management of APS requires early diagnosis and identification of traditional risk factors for atherosclerosis, including obesity, diabetes mellitus, hypertension, hyperlipidemia, and smoking. Common strategies for the treatment and prevention of antiphospholipid syndrome mainly focus on low-dose aspirin, Vitamin K antagonists, and heparin. However, these medications are usually ineffective for microvasculature and non-thrombotic manifestations. 12 Data on the use of immunosuppressive strategies in patients with difficult-to-treat APS are limited. Commonly used immunosuppressive strategies include B-cell inhibition, complement inhibition, mammalian target of rapamycin [mTOR] inhibition, and hydroxychloroquine (HCQ).12 Vitamin K antagonists, such as warfarin, are the current standard treatment for unprovoked thrombosis. For prevention of recurrent obstetric complications, usually low-dose aspirin and prophylactic heparin such as low-molecular-weight heparin are used.13
5. Conclusion
Antiphospholipid antibody syndrome can present in a typical or atypical manner; thus, we must keep a number of differential diagnoses on our list before ruling out APS. It can cause thrombosis in any organ because of the highly thrombotic nature of the disease. Primary care physicians must recognize and aggressively control the traditional risk factors to prevent further endothelial and vascular damage. The diagnosis of this disease is based on laboratory work-up and clinical findings. Treatment options for antiphospholipid antibody syndrome include low-dose aspirin, warfarin, and heparin. Long-term management of antiphospholipid antibody syndrome involves the use of warfarin and the control of traditional risk factors.
Acknowledgments
We would like to acknowledge Priyanka Bhatia, MD, and Kyunghyun Lee, MD, for their supervision and assistance in this case.
Footnotes
Consent: Informed consent was obtained from the patient for publication of this case and accompanying images.
Conflict of interest: The authors declare that they have no conflict of interest.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Garcia D, Erkan D. Diagnosis and Management of the antiphospholipid syndrome. N Engl J Med. 2018;378(21):2010–2021. doi: 10.1056/NEJMra1705454. [DOI] [PubMed] [Google Scholar]
- 2. Kadeli DK, Hanjagi SY. Primary antiphospholipid antibody syndrome: a case report. J Clin Diagn Res. 2015;9(10):OD04–OD5. doi: 10.7860/JCDR/2015/10236.6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Parsi M, Rai M, Swaab R. A rare case of catastrophic antiphospholipid antibody syndrome: a case report and review of traditional cardiovascular risk factors implicated in disease occurrence. Cureus. 2020;12(3):e7221. doi: 10.7759/cureus.7221. Published 2020 Mar 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lekovic JP, Friedman CM, Desancho MT. Lingual thrombosis in a woman with antiphospholipid syndrome. Am J Obstet Gynecol. 2013;208(4):e3–e4. doi: 10.1016/j.ajog.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 5. Zaragoza JR, Vernon N, Ghaffari G. Tongue necrosis as an initial manifestation of giant cell arteritis: case report and review of the literature. Case Rep Rheumatol. 2015;2015:901795. doi: 10.1155/2015/901795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kamatani T, Yamashita K, Okabayashi T, Maeda H, Toi M, Yamamoto T. Bilateral ischemic necrosis of the tongue due to disseminated intravascular coagulation. Int J OralMaxillofac Surg. 2008;37(8):777–779. doi: 10.1016/j.ijom.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 7. Bjordahl PM, Ammar AD. Tongue necrosis as an unusual presentation of carotid artery stenosis. J Vasc Surg. 2011;54(3):837–839. doi: 10.1016/j.jvs.2011.01.057. [DOI] [PubMed] [Google Scholar]
- 8. Pfeiffer J, Ridder GJ. Spontane Zungennekrose bei rheumatoidem Hyperviskositätssyndrom. Fallbericht und Literaturübersicht. [Spontaneous tongue necrosis consecutive to rheumatoid hyperviscosity syndrome. A case report and literature review]. Laryngo-Rhino-Otol. 2008;87(1):43–48. doi: 10.1055/s-2007-966779. [DOI] [PubMed] [Google Scholar]
- 9. Roman BR, Immerman SB, Morris LG. Ischemic necrosis of the tongue in patients with cardiogenic shock. Laryngoscope. 2010;120(7):1345–1349. doi: 10.1002/lary.20974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Noordally SO, Sohawon S, Duttmann R, Gottignies P, Devriendt J. Tongue necrosis as a complication of vasoconstrictor agents in the intensive care setting. Intern Emerg Med. 2011;6(2):183–185. doi: 10.1007/s11739-010-0416-3. [DOI] [PubMed] [Google Scholar]
- 11. Gardiner C, Hills J, Machin SJ, Cohen H. Diagnosis of antiphospholipid syndrome in routine clinical practice. Lupus. 2013;22(1):18–25. doi: 10.1177/0961203312460722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Erton ZB, Erkan D. Treatment advances in antiphospholipid syndrome: 2022 update. Curr Opin Pharmacol. 2022;65:102212. doi: 10.1016/j.coph.2022.102212. [DOI] [PubMed] [Google Scholar]
- 13. Sammaritano LR. Antiphospholipid syndrome. Best Pract Res Clin Rheumatol. 2020;34(1):101463. doi: 10.1016/j.berh.2019.101463. [DOI] [PubMed] [Google Scholar]
- 14. Giannakopoulos B, Krilis SA. The pathogenesis of the antiphospholipid syndrome. N Engl J Med. 2013;368(11):1033–1044. doi: 10.1056/NEJMra1112830. [DOI] [PubMed] [Google Scholar]