FIGURE 8.
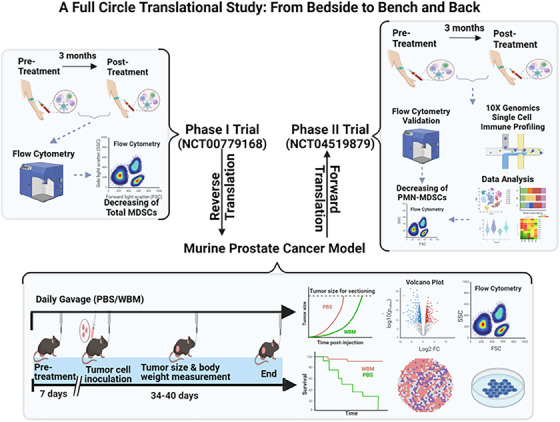
A Full circle translational research design to explore the immunoregulatory effects of WBM in PCa patients. The initial single‐arm Phase I clinical trial involving WBM tablet consumption (NCT00779168) in PCa patients identified circulating MDSCs as immune cells responsive to WBM treatment. Building upon these findings, the current study includes experiments on PCa murine models and analysis of PCa patients in a dual‐centre, two‐arm, open‐label, randomised Phase II trial (NCT04519879). This bidirectional translational research approach has supported the immunomodulatory role of WBM on MDSCs, thus strengthening the rationale for using WBM as a nutraceutical approach to slow the progression of prostate cancer.
