Abstract
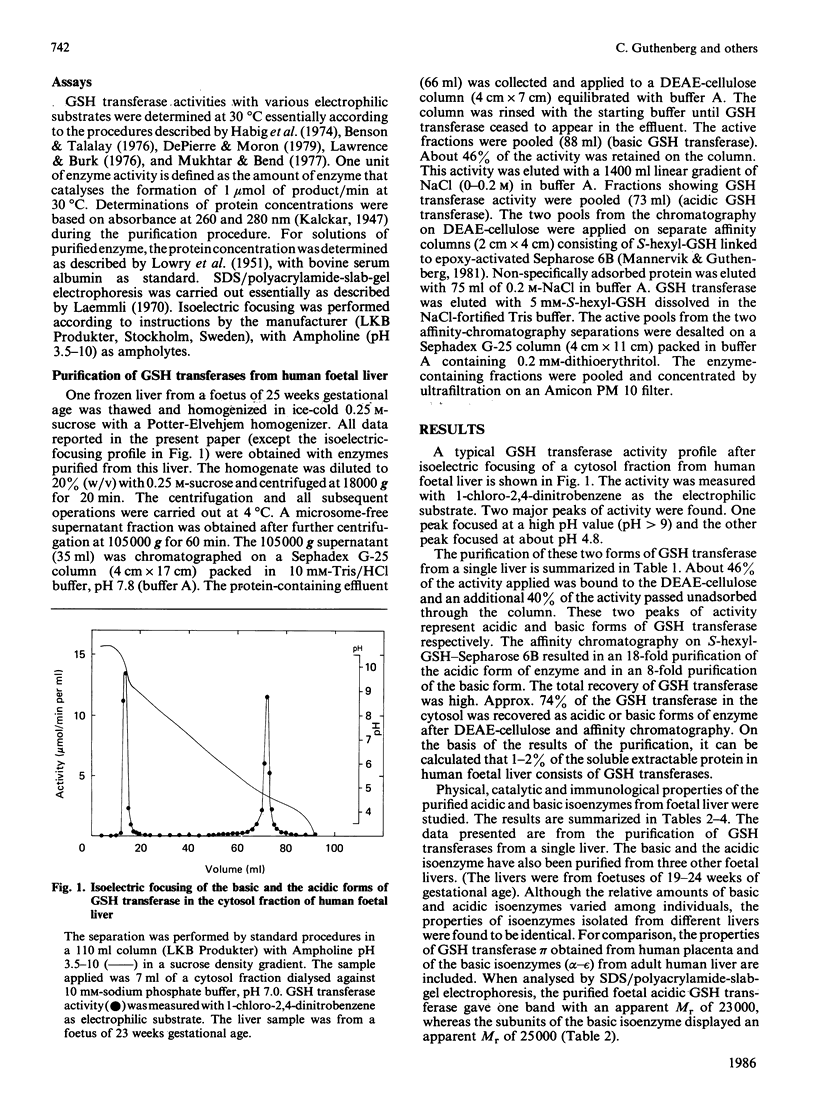
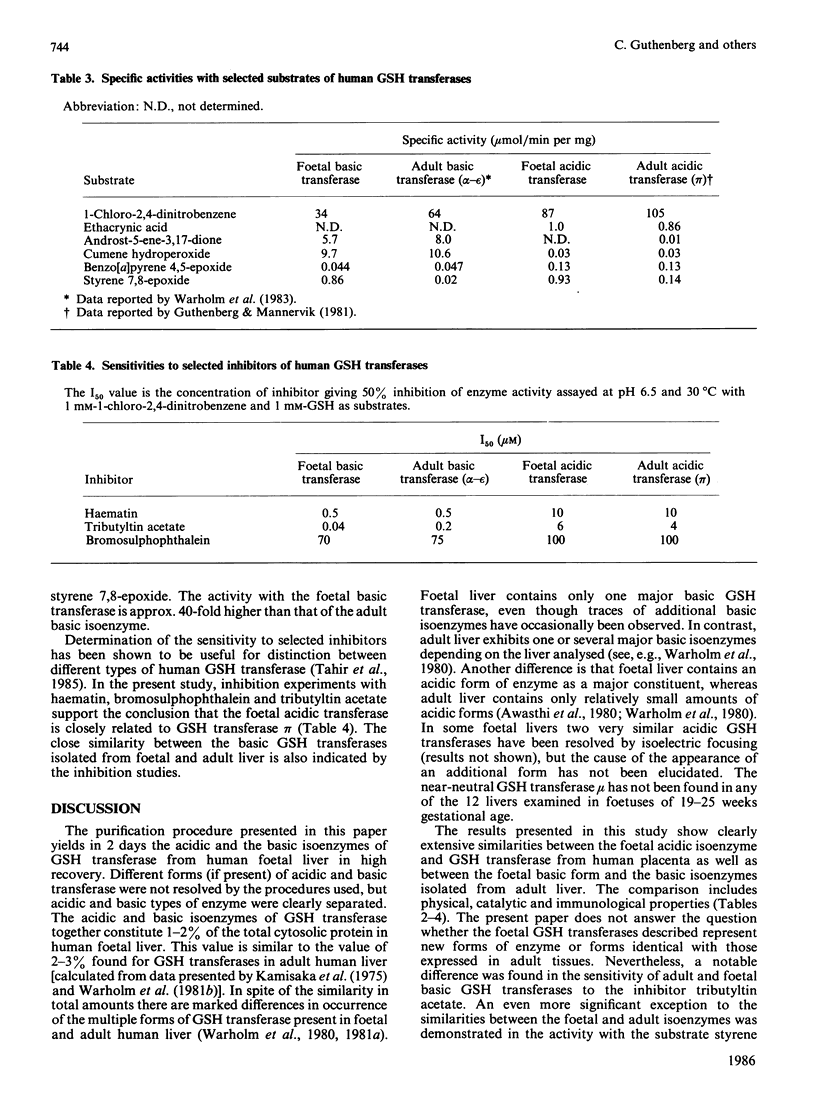
Isoelectric focusing of a cytosol fraction from human foetal liver revealed the existence of an acidic and a basic isoenzyme of GSH transferase. The acidic and basic forms of GSH transferase were purified in good yield by use of ion-exchange chromatography on DEAE-cellulose followed by affinity chromatography on S-hexyl-GSH coupled to epoxy-activated Sepharose 6B. The content of the acidic and the basic isoenzymes of GSH transferase together was calculated to constitute 1-2% of the soluble proteins in the hepatic cytoplasm. Physical, catalytic and immunological analyses of the acidic and the basic isoenzymes from foetal liver demonstrated unambiguously that the two forms are different structures with distinct properties. On the other hand, the results show clearly extensive similarities between the foetal acidic transferase and transferase pi from human placenta as well as between the foetal basic form and the basic isoenzymes isolated from adult liver. An exception is that both foetal enzymes seem to be considerably more efficient in catalysing the conjugation of GSH with styrene 7,8-epoxide than the corresponding adult forms of GSH transferase.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alin P., Mannervik B., Jörnvall H. Structural evidence for three different types of glutathione transferase in human tissues. FEBS Lett. 1985 Mar 25;182(2):319–322. doi: 10.1016/0014-5793(85)80324-0. [DOI] [PubMed] [Google Scholar]
- Awasthi Y. C., Dao D. D., Saneto R. P. Interrelationship between anionic and cationic forms of glutathione S-transferases of human liver. Biochem J. 1980 Oct 1;191(1):1–10. doi: 10.1042/bj1910001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson A. M., Talalay P. Role of reduced glutathione in the delta(5)-3-kitosteroid isomerase reaction of liver. Biochem Biophys Res Commun. 1976 Apr 19;69(4):1073–1079. doi: 10.1016/0006-291x(76)90482-4. [DOI] [PubMed] [Google Scholar]
- Chasseaud L. F. The role of glutathione and glutathione S-transferases in the metabolism of chemical carcinogens and other electrophilic agents. Adv Cancer Res. 1979;29:175–274. doi: 10.1016/s0065-230x(08)60848-9. [DOI] [PubMed] [Google Scholar]
- DePierre J. W., Moron M. S. Assaying glutathione S-transferase activity in lung and liver with 3H-styrene oxide as substrate. Pharmacol Res Commun. 1979 May;11(5):421–431. doi: 10.1016/s0031-6989(79)80006-5. [DOI] [PubMed] [Google Scholar]
- Guthenberg C., Akerfeldt K., Mannervik B. Purification of glutathione-S-transferase from human placenta. Acta Chem Scand B. 1979;33(8):595–596. doi: 10.3891/acta.chem.scand.33b-0595. [DOI] [PubMed] [Google Scholar]
- Guthenberg C., Mannervik B. Glutathione S-transferase (transferase pi) from human placenta is identical or closely related to glutathione S-transferase (transferase rho) from erythrocytes. Biochim Biophys Acta. 1981 Oct 13;661(2):255–260. doi: 10.1016/0005-2744(81)90012-7. [DOI] [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974 Nov 25;249(22):7130–7139. [PubMed] [Google Scholar]
- Kamisaka K., Habig W. H., Ketley J. N., Arias M., Jakoby W. B. Multiple forms of human glutathione S-transferase and their affinity for bilirubin. Eur J Biochem. 1975 Dec 1;60(1):153–161. doi: 10.1111/j.1432-1033.1975.tb20987.x. [DOI] [PubMed] [Google Scholar]
- Koskelo K. Isoelectric focusing of glutathione S-transferases: comparison of the acidic transferases from human liver, kidney, lung, spleen and placenta. Scand J Clin Lab Invest. 1983 Apr;43(2):133–139. doi: 10.1080/00365518309168235. [DOI] [PubMed] [Google Scholar]
- Koskelo K., Valmet E. Acid glutathione S-transferase from human liver: preliminary report. Scand J Clin Lab Invest. 1980 Apr;40(2):179–184. doi: 10.3109/00365518009093023. [DOI] [PubMed] [Google Scholar]
- Koskelo K., Valmet E., Tenhunen R. Purification and characterization of an acid glutathione S-transferase from human lung. Scand J Clin Lab Invest. 1981 Nov;41(7):683–689. doi: 10.3109/00365518109090515. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawrence R. A., Burk R. F. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun. 1976 Aug 23;71(4):952–958. doi: 10.1016/0006-291x(76)90747-6. [DOI] [PubMed] [Google Scholar]
- Mannervik B., Guthenberg C. Glutathione transferase (human placenta). Methods Enzymol. 1981;77:231–235. doi: 10.1016/s0076-6879(81)77030-7. [DOI] [PubMed] [Google Scholar]
- Mannervik B. The isoenzymes of glutathione transferase. Adv Enzymol Relat Areas Mol Biol. 1985;57:357–417. doi: 10.1002/9780470123034.ch5. [DOI] [PubMed] [Google Scholar]
- Marcus C. J., Habig W. H., Jakoby W. B. Glutathione transferase from human erythrocytes. Nonidentity with the enzymes from liver. Arch Biochem Biophys. 1978 Jun;188(2):287–293. doi: 10.1016/s0003-9861(78)80011-3. [DOI] [PubMed] [Google Scholar]
- Mukhtar H., Bend J. R. Serum glutathione S-transferases: perinatal development, sex difference, and effect of carbon tetrachloride administration on enzyme activity in the rat. Life Sci. 1977 Nov 1;21(9):1277–1286. doi: 10.1016/0024-3205(77)90008-x. [DOI] [PubMed] [Google Scholar]
- Polidoro G., Di Ilio C., Del Boccio G., Zulli P., Federici G. Glutathione S-transferase activity in human placenta. Biochem Pharmacol. 1980 Jun 15;29(12):1677–1680. doi: 10.1016/0006-2952(80)90124-0. [DOI] [PubMed] [Google Scholar]
- Stockman P. K., Beckett G. J., Hayes J. D. Identification of a basic hybrid glutathione S-transferase from human liver. Glutathione S-transferase delta is composed of two distinct subunits (B1 and B2). Biochem J. 1985 Apr 15;227(2):457–465. doi: 10.1042/bj2270457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahir M. K., Guthenberg C., Mannervik B. Inhibitors for distinction of three types of human glutathione transferase. FEBS Lett. 1985 Feb 25;181(2):249–252. doi: 10.1016/0014-5793(85)80269-6. [DOI] [PubMed] [Google Scholar]
- Vander Jagt D. L., Dean V. L., Wilson S. P., Royer R. E. Regulation of the glutathione S-transferase activity of bilirubin transport protein (ligandin) from human liver. Enzymic memory involving protein-protein interactions. J Biol Chem. 1983 May 10;258(9):5689–5694. [PubMed] [Google Scholar]
- Vander Jagt D. L., Hunsaker L. A., Garcia K. B., Royer R. E. Isolation and characterization of the multiple glutathione S-transferases from human liver. Evidence for unique heme-binding sites. J Biol Chem. 1985 Sep 25;260(21):11603–11610. [PubMed] [Google Scholar]
- Warholm M., Guthenberg C., Mannervik B. Molecular and catalytic properties of glutathione transferase mu from human liver: an enzyme efficiently conjugating epoxides. Biochemistry. 1983 Jul 19;22(15):3610–3617. doi: 10.1021/bi00284a011. [DOI] [PubMed] [Google Scholar]
- Warholm M., Guthenberg C., Mannervik B., Pacifici G. M., Rane A. Glutathione S-transferases in human fetal liver. Acta Chem Scand B. 1981;35(3):225–227. doi: 10.3891/acta.chem.scand.35b-0225. [DOI] [PubMed] [Google Scholar]
- Warholm M., Guthenberg C., Mannervik B., von Bahr C., Glaumann H. Identification of a new glutathione S-transferase in human liver. Acta Chem Scand B. 1980;34(8):607–621. doi: 10.3891/acta.chem.scand.34b-0607. [DOI] [PubMed] [Google Scholar]
- Warholm M., Guthenberg C., Mannervik B., von Bahr C. Purification of a new glutathione S-transferase (transferase mu) from human liver having high activity with benzo(alpha)pyrene-4,5-oxide. Biochem Biophys Res Commun. 1981 Jan 30;98(2):512–519. doi: 10.1016/0006-291x(81)90870-6. [DOI] [PubMed] [Google Scholar]



