Fig. 1.
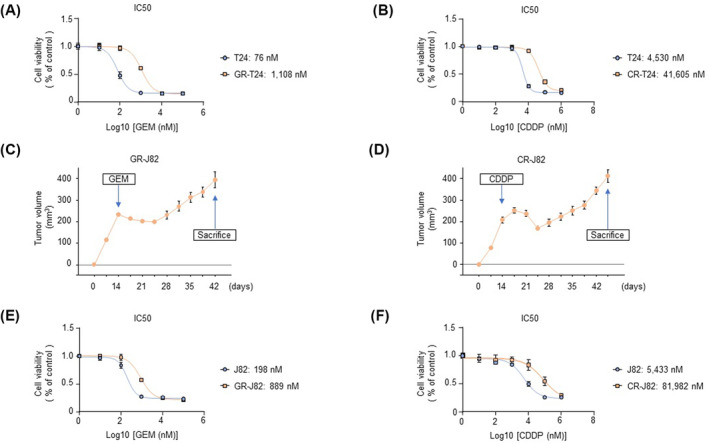
Establishment of gemcitabine‐ and cisplatin‐resistant cells (T24 and J82). (A) IC50 values of parental T24 and gemcitabine‐resistant T24 cells (n = 3). The error bars indicate SEM. (B) IC50 values of parental T24 and cisplatin‐resistant T24 cells (n = 3). The error bars indicate SEM. (C) Establishment of gemcitabine‐resistant J82 cells in vivo. The tumor volume in mice subcutaneously injected with parental J82 cells after treatment with gemcitabine (150 mg·kg−1, once a week) is shown (n = 3). The error bars indicate SEM. (D) Establishment of cisplatin‐resistant J82 cells in vivo (n = 3). The tumor volume in mice subcutaneously injected with parental J82 cells after treatment with cisplatin (4 mg·kg−1, five times a week) is shown (n = 3). The error bars indicate SEM. (E) IC50 values of parental J82 and gemcitabine‐resistant J82 cells treated with gemcitabine (n = 3). The error bars indicate SEM. (F) IC50 values of parental J82 and cisplatin‐resistant J82 cells treated with cisplatin (n = 3). The error bars indicate SEM. CDDP, cisplatin; CR‐J82, cisplatin‐resistant J82; CR‐T24, cisplatin‐resistant T24; GEM, gemcitabine; GR‐J82, gemcitabine‐resistant J82; GR‐T24, gemcitabine‐resistant T24; IC50, half maximal (50%) inhibitory concentration.
