Figure 8.
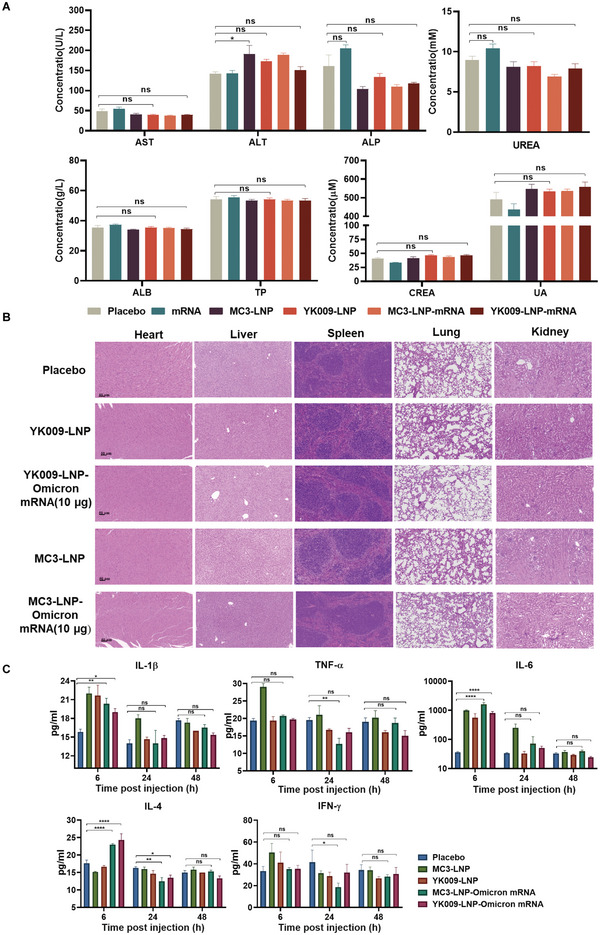
In vivo safety evaluation of LNP formulations. A) Serum level of aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), UREA, albumin (ALB), total protein (TP), creatinine (CREA), and uric acid (UA) in female BALB/c mice that were vaccinated with placebo, Omicron mRNA, MC3‐LNP, YK009‐LNP, MC3‐LNP‐Omicron mRNA, or YK009‐LNP‐Omicron mRNA (mRNA = 10 µg) after 24 h. Significance was calculated by one‐way ANOVA and data are presented as mean ± SEM (n = 6, ns: no significant difference, *P < 0.05, **P < 0.01). B) Histopathological examination (H&E) of the heart, liver, spleen, lungs, and kidneys at 48 h postinjection with placebo, MC3‐LNP, YK009‐LNP, MC3‐LNP‐Omicron mRNA, and YK009‐LNP‐Omicron mRNA (mRNA = 10 µg). C) Cytokines in the serum at 6, 24, and 48 h postinjection. Statistical significance was calculated by a two‐way ANOVA analysis followed by Dunnett's multiple comparison tests. Data are shown as mean ± SEM (n = 3, ns: no significant difference, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001).
