Figure 5.
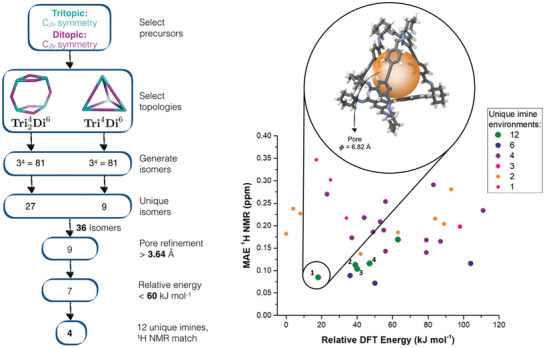
(Left) Computational pipeline for generating and screening potential structural isomers, which in combination with experimental data filtered down to four plausible candidate isomers. (Right) Comparison of the 36 unique isomers taking into account the relative DFT energy, the number of unique imine environments, and the mean absolute error (MAE) between the experimental and calculated 1H NMR spectra. The final four candidate isomers are labeled, with the structure of the proposed isomer having the lowest relative energy and the lowest MAE of the 1H NMR shifts, shown. Reproduced with permission.[ 14 ] Copyright 2018, The Royal Society of Chemistry.
