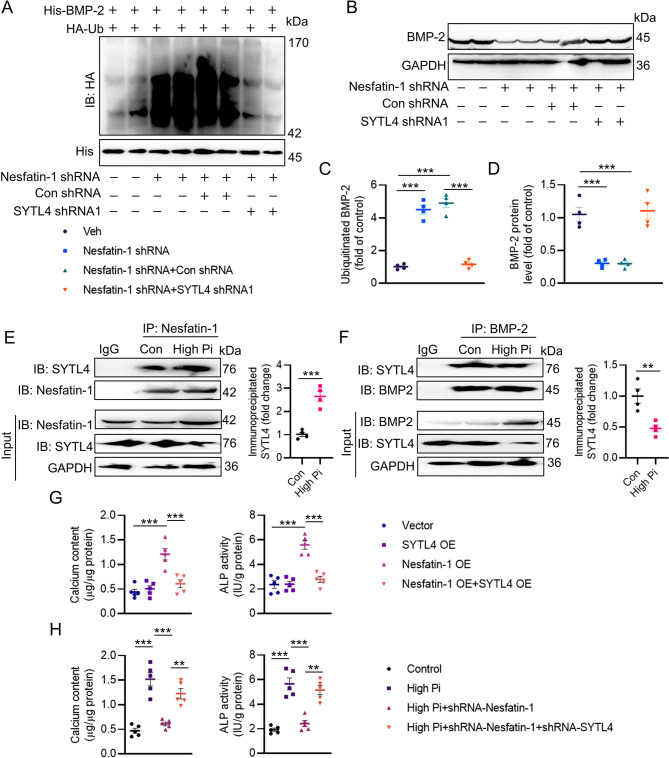
Fig. 6.
SYTL4 contributes to BMP-2 ubiquitination and degradation. (A, C) Silencing SYTL4 abolished the effects of nesfatin-1 knockdown on the ubiquitination of BMP-2. (B, D) Silencing SYTL4 abolished the effects of nesfatin-1 knockdown on the protein expression of BMP-2. (E) High Pi increased the interaction of nesfatin-1 with SYTL4. (F) High Pi decreased the interaction of BMP-2 with SYTL4. (G) Overexpression of SYTL4 attenuated the deposition of calcium and ALP activity induced by nesfatin-1 overexpression. (H) Downregulation of SYTL4 attenuated the effects of nesfatin-1 deficiency on calcium deposition and ALP activity in VSMCs exposed to high Pi. Differences between groups were assessed with ANOVA followed by Bonferroni post-hoc test (C-D, G-H). The P-value was calculated by unpaired two-tailed Student’s t-test (E-F). *P < 0.05, **P < 0.01, ***P < 0.001 versus the indicated group. n = 6