Abstract
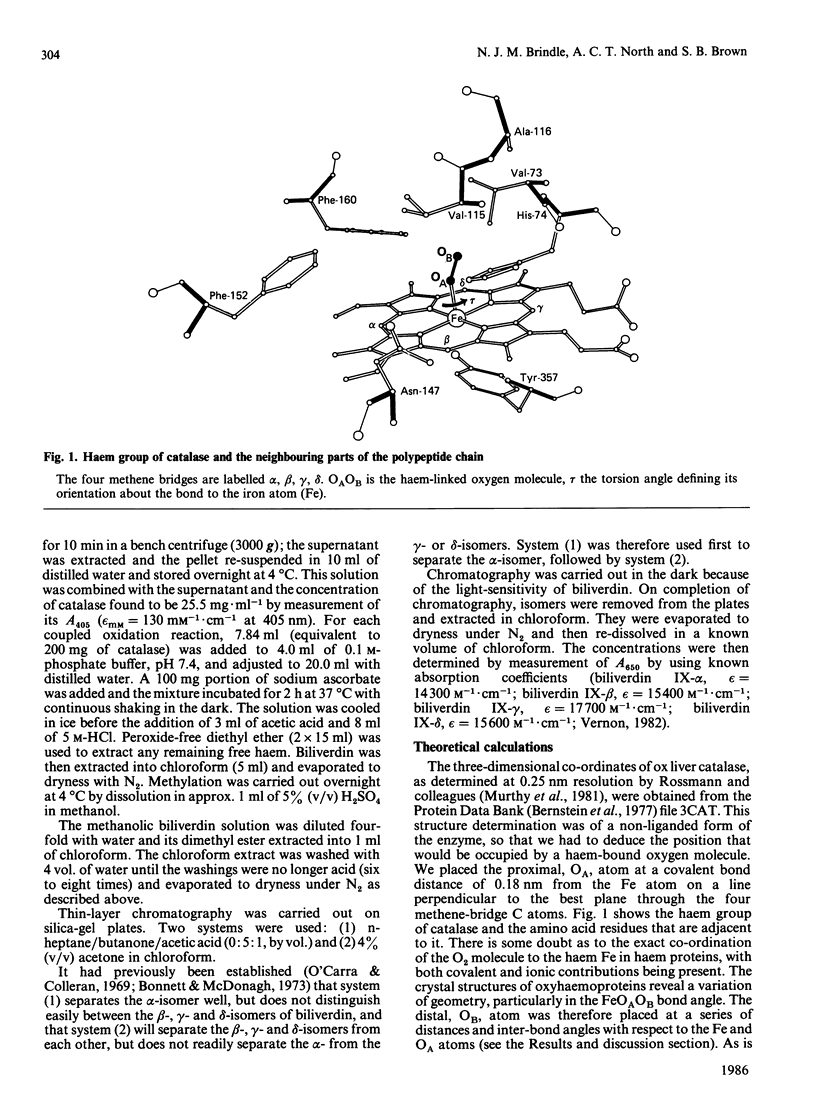
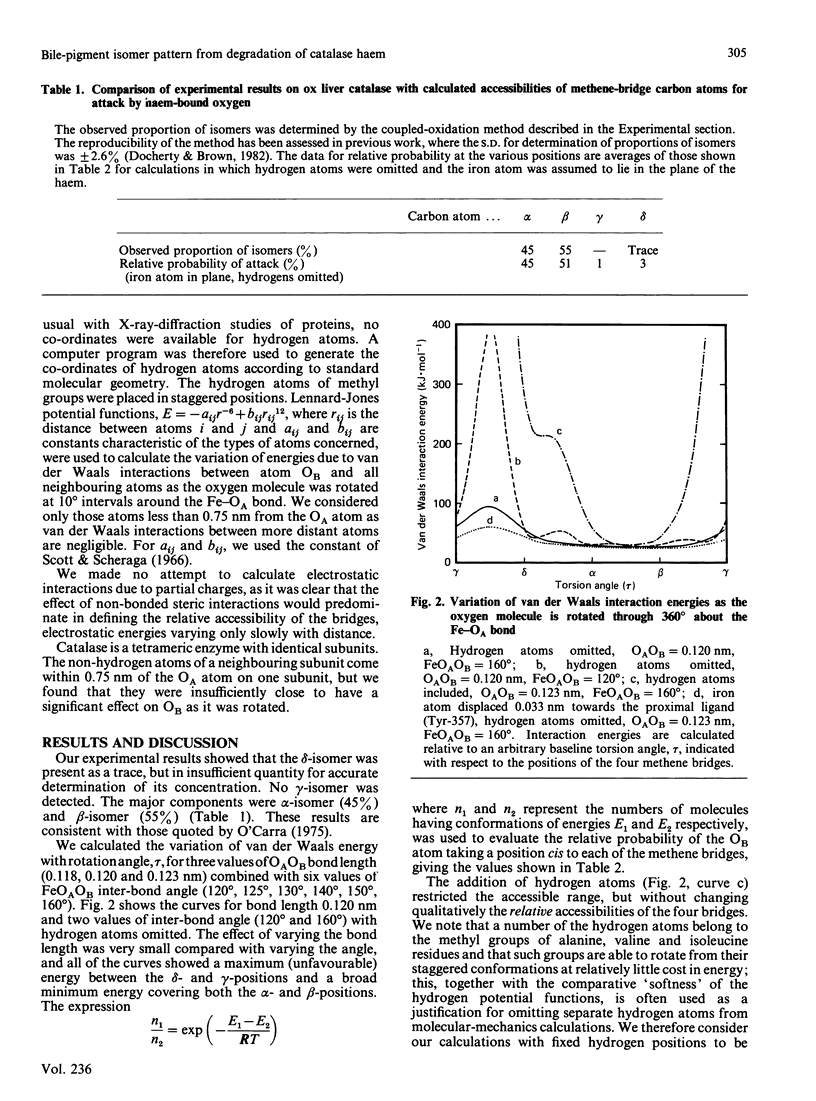
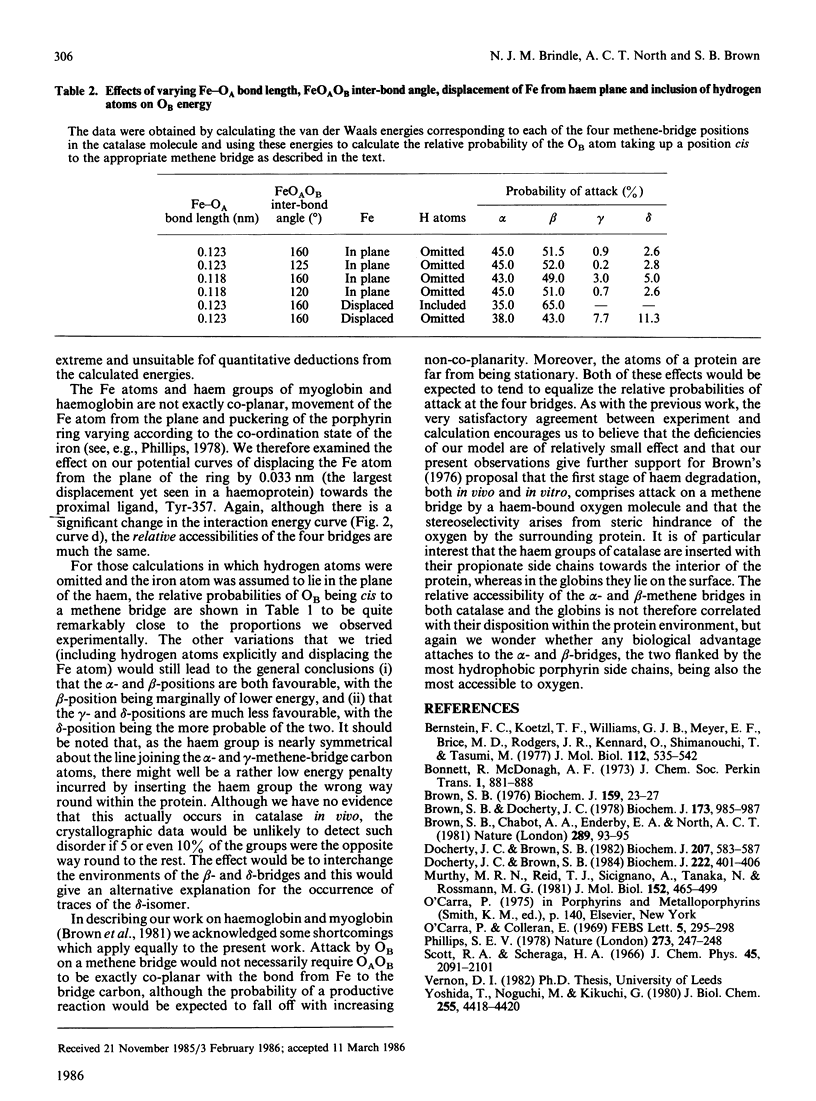
Degradation in vitro of the haem in catalase by a 'coupled oxidation' reaction yields products in which approx. 45% of the haem groups have been cleaved at the alpha-methene bridge, 55% at the beta-bridge and a trace at the delta-bridge. Molecular-mechanics calculations with the three-dimensional structural co-ordinates of catalase shows that these proportions of products can be accounted for by the relative accessibility of the four methene bridges to a haem-linked oxygen molecule, thus further confirming Brown's [(1976) Biochem. J. 159, 23-27] hypothesis that the first stage of haem catabolism in vivo is selective attack by haem-bound oxygen, with selectivity conferred by the surrounding protein moiety.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- 'Carra P. O., Colleran E. HAEM catabolism and coupled oxidation of haemproteins. FEBS Lett. 1969 Nov 29;5(4):295–298. doi: 10.1016/0014-5793(69)80372-8. [DOI] [PubMed] [Google Scholar]
- Bernstein F. C., Koetzle T. F., Williams G. J., Meyer E. F., Jr, Brice M. D., Rodgers J. R., Kennard O., Shimanouchi T., Tasumi M. The Protein Data Bank: a computer-based archival file for macromolecular structures. J Mol Biol. 1977 May 25;112(3):535–542. doi: 10.1016/s0022-2836(77)80200-3. [DOI] [PubMed] [Google Scholar]
- Bonnett R., McDonagh A. F. The meso-reactivity of porphyrins and related compounds. VI. Oxidative cleavage of the haem system. The four isomeric biliverdins of the IX series. J Chem Soc Perkin 1. 1973;9:881–888. doi: 10.1039/p19730000881. [DOI] [PubMed] [Google Scholar]
- Brown S. B., Chabot A. A., Enderby E. A., North A. C. Orientation of oxygen in oxyhaemoproteins and its implications for haem catabolism. Nature. 1981 Jan 1;289(5793):93–95. doi: 10.1038/289093a0. [DOI] [PubMed] [Google Scholar]
- Brown S. B., Docherty J. C. Haem degradation in abnormal haemoglobins. Biochem J. 1978 Sep 1;173(3):985–987. doi: 10.1042/bj1730985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. B. Stereospecific haem cleavage. A model for the formation of bile-pigment isomers in vivo and in vitro. Biochem J. 1976 Oct 1;159(1):23–27. doi: 10.1042/bj1590023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty J. C., Brown S. B. Haem degradation in human haemoglobin in vitro. Separation of the contribution of the alpha- and beta-subunits. Biochem J. 1984 Sep 1;222(2):401–406. doi: 10.1042/bj2220401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty J. C., Brown S. B. Haem disorder in reconstituted human haemoglobin. Biochem J. 1982 Dec 1;207(3):583–587. doi: 10.1042/bj2070583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy M. R., Reid T. J., 3rd, Sicignano A., Tanaka N., Rossmann M. G. Structure of beef liver catalase. J Mol Biol. 1981 Oct 25;152(2):465–499. doi: 10.1016/0022-2836(81)90254-0. [DOI] [PubMed] [Google Scholar]
- Phillips S. E. Structure of oxymyoglobin. Nature. 1978 May 18;273(5659):247–248. doi: 10.1038/273247a0. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Noguchi M., Kikuchi G. Oxygenated form of heme . heme oxygenase complex and requirement for second electron to initiate heme degradation from the oxygenated complex. J Biol Chem. 1980 May 25;255(10):4418–4420. [PubMed] [Google Scholar]


