Abstract
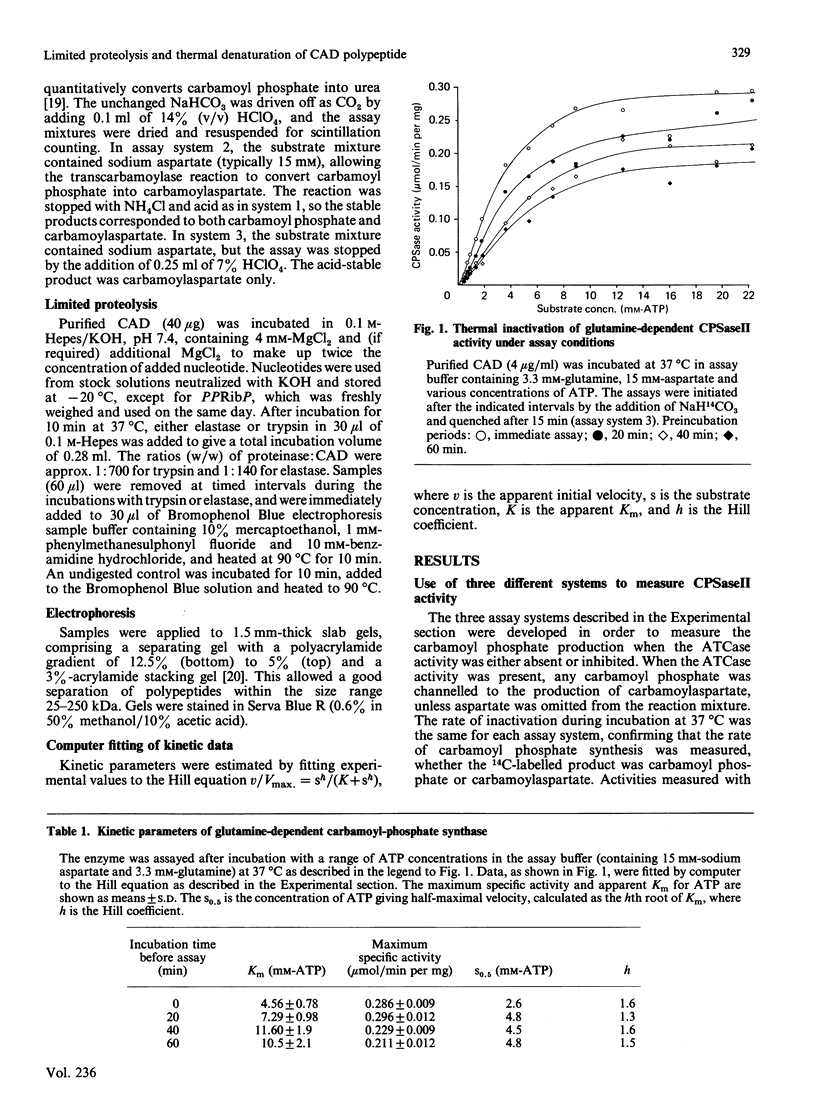
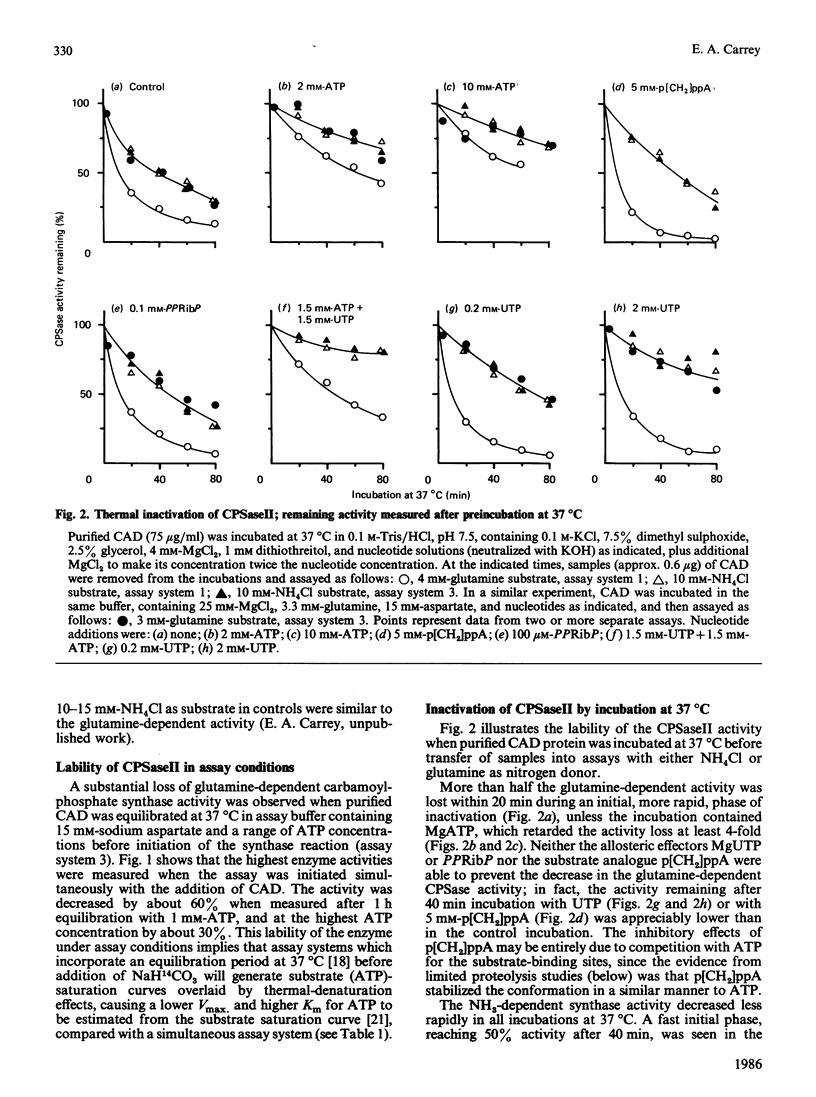
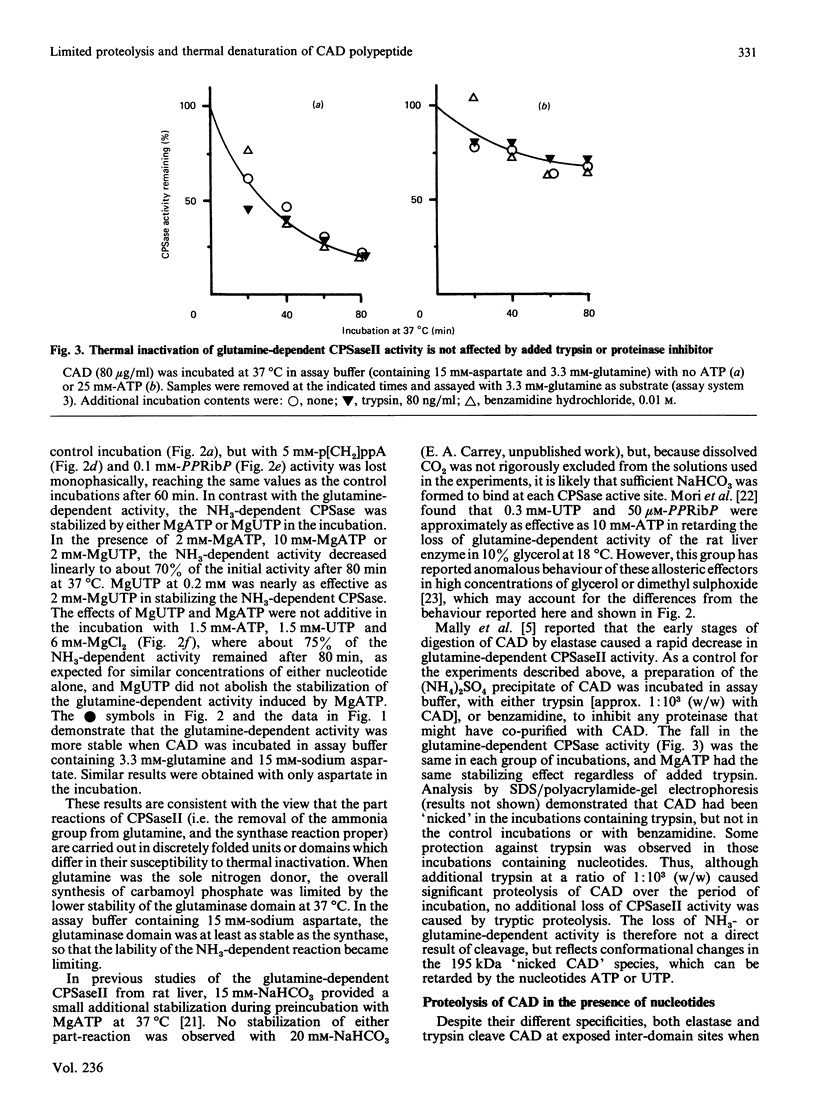
Improved methodologies are described which allow the measurement of the part-reactions, with glutamine or ammonia as nitrogen donor, of mammalian carbamoyl-phosphate synthase II (EC 6.3.5.5) through the incorporation of [14C]bicarbonate into either carbamoyl phosphate or carbamoylaspartate. The enzyme is part of the multifunctional polypeptide (CAD) which also comprises the pyrimidine-biosynthetic enzymes aspartate transcarbamoylase (EC 2.1.3.2) and dihydro-orotase (EC 3.5.2.3). The conformational stability of the carbamoyl-phosphate synthase was investigated through the inactivation of the part-reactions which occurred during incubation at 37 degrees C. The domain involved in the removal of the amide N from glutamine was more thermolabile than the ammonia-dependent synthase moiety. The former activity was stabilized in the presence of sodium aspartate or MgATP, whereas the latter was stabilized by MgATP and MgUTP. Binding of MgUTP and MgATP to CAD restricted the initial proteolysis by trypsin and elastase of one or both regions linking the carbamoyl-phosphate synthase domain to the other major domains. A model is described to account for both aspects of nucleotide binding to CAD; these stabilizing effects may be important in the cell, where similar concentrations of nucleotides are found.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babul J., Stellwagen E. Measurement of protein concentration with interferences optics. Anal Biochem. 1969 Apr 4;28(1):216–221. doi: 10.1016/0003-2697(69)90172-9. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Coleman P. F., Suttle D. P., Stark G. R. Purification from hamster cells of the multifunctional protein that initiates de novo synthesis of pyrimidine nucleotides. J Biol Chem. 1977 Sep 25;252(18):6379–6385. [PubMed] [Google Scholar]
- Davidson J. N., Patterson D. Alteration in structure of multifunctional protein from Chinese hamster ovary cells defective in pyrimidine biosynthesis. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1731–1735. doi: 10.1073/pnas.76.4.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaney M. A., Powers-Lee S. G. Immunological cross-reactivity between carbamyl phosphate synthetases I, II, and III. J Biol Chem. 1984 Jan 25;259(2):703–706. [PubMed] [Google Scholar]
- Grayson D. R., Evans D. R. The isolation and characterization of the aspartate transcarbamylase domain of the multifunctional protein, CAD. J Biol Chem. 1983 Apr 10;258(7):4123–4129. [PubMed] [Google Scholar]
- Ishida H., Mori M., Tatibana M. Effects of dimethyl sulfoxide and glycerol on catalytic and regulatory properties of glutamine-dependent carbamoyl phosphate synthase from rat liver and dual effects of uridine triphosphate. Arch Biochem Biophys. 1977 Jul;182(1):258–265. doi: 10.1016/0003-9861(77)90306-x. [DOI] [PubMed] [Google Scholar]
- Jackson R. C., Lui M. S., Boritzki T. J., Morris H. P., Weber G. Purine and pyrimidine nucleotide patterns of normal, differentiating, and regenerating liver and of hepatomas in rats. Cancer Res. 1980 Apr;40(4):1286–1291. [PubMed] [Google Scholar]
- Jones M. E. Pyrimidine nucleotide biosynthesis in animals: genes, enzymes, and regulation of UMP biosynthesis. Annu Rev Biochem. 1980;49:253–279. doi: 10.1146/annurev.bi.49.070180.001345. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lue P. F., Kaplan J. G. The aspartate transcarbamylase and carbamoyl phosphate synthetase of yeast: a multi-functional enzyme complex. Biochem Biophys Res Commun. 1969 Feb 21;34(4):426–433. doi: 10.1016/0006-291x(69)90399-4. [DOI] [PubMed] [Google Scholar]
- Lyons S. D., Christopherson R. I. Regulation of hamster carbamoyl-phosphate synthase II by 5-phospho-alpha-D-ribosyl 1-diphosphate and uridine 5'-triphosphate. Eur J Biochem. 1985 Mar 15;147(3):587–592. doi: 10.1111/j.0014-2956.1985.00587.x. [DOI] [PubMed] [Google Scholar]
- Makoff A. J., Buxton F. P., Radford A. A possible model for the structure of the Neurospora carbamoyl phosphate synthase-aspartate carbamoyl transferase complex enzyme. Mol Gen Genet. 1978 May 31;161(3):297–304. doi: 10.1007/BF00331004. [DOI] [PubMed] [Google Scholar]
- Mally M. I., Grayson D. R., Evans D. R. Catalytic synergy in the multifunctional protein that initiates pyrimidine biosynthesis in Syrian hamster cells. J Biol Chem. 1980 Dec 10;255(23):11372–11380. [PubMed] [Google Scholar]
- Mally M. I., Grayson D. R., Evans D. R. Controlled proteolysis of the multifunctional protein that initiates pyrimidine biosynthesis in mammalian cells: evidence for discrete structural domains. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6647–6651. doi: 10.1073/pnas.78.11.6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M., Ishida H., Tatibana M. Aggregation states and catalytic properties of the multienzyme complex catalyzing the initial steps of pyrimidine biosynthesis in rat liver. Biochemistry. 1975 Jun 17;14(12):2622–2630. doi: 10.1021/bi00683a010. [DOI] [PubMed] [Google Scholar]
- Nyunoya H., Broglie K. E., Lusty C. J. The gene coding for carbamoyl-phosphate synthetase I was formed by fusion of an ancestral glutaminase gene and a synthetase gene. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2244–2246. doi: 10.1073/pnas.82.8.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyunoya H., Broglie K. E., Widgren E. E., Lusty C. J. Characterization and derivation of the gene coding for mitochondrial carbamyl phosphate synthetase I of rat. J Biol Chem. 1985 Aug 5;260(16):9346–9356. [PubMed] [Google Scholar]
- Rigby D. J., Radford A. Investigation of binding sites in the complex pyrimidine-specific carbamoyl-phosphate synthetase/aspartate carbamoyltransferase enzyme of Neurospora crassa. Biochim Biophys Acta. 1982 Dec 20;709(2):154–159. doi: 10.1016/0167-4838(82)90455-1. [DOI] [PubMed] [Google Scholar]
- Rumsby P. C., Campbell P. C., Niswander L. A., Davidson J. N. Organization of a multifunctional protein in pyrimidine biosynthesis. A domain hypersensitive to proteolysis. Biochem J. 1984 Jan 15;217(2):435–440. doi: 10.1042/bj2170435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatibana M., Shigesada K. Control of pyrimidine biosynthesis in mammalian tissues. IV. Requirements of a quantitative assay of glutamine-dependent carbamyl phosphate synthetase and effect of magnesium ion as an essential activator. J Biochem. 1972 Sep;72(3):537–547. doi: 10.1093/oxfordjournals.jbchem.a129933. [DOI] [PubMed] [Google Scholar]
- YASHPHE J., GORINI L. PHOSPHORYLATION OF CARBAMATE IN VIVO AND IN VITRO. J Biol Chem. 1965 Apr;240:1681–1686. [PubMed] [Google Scholar]