Figure 2.
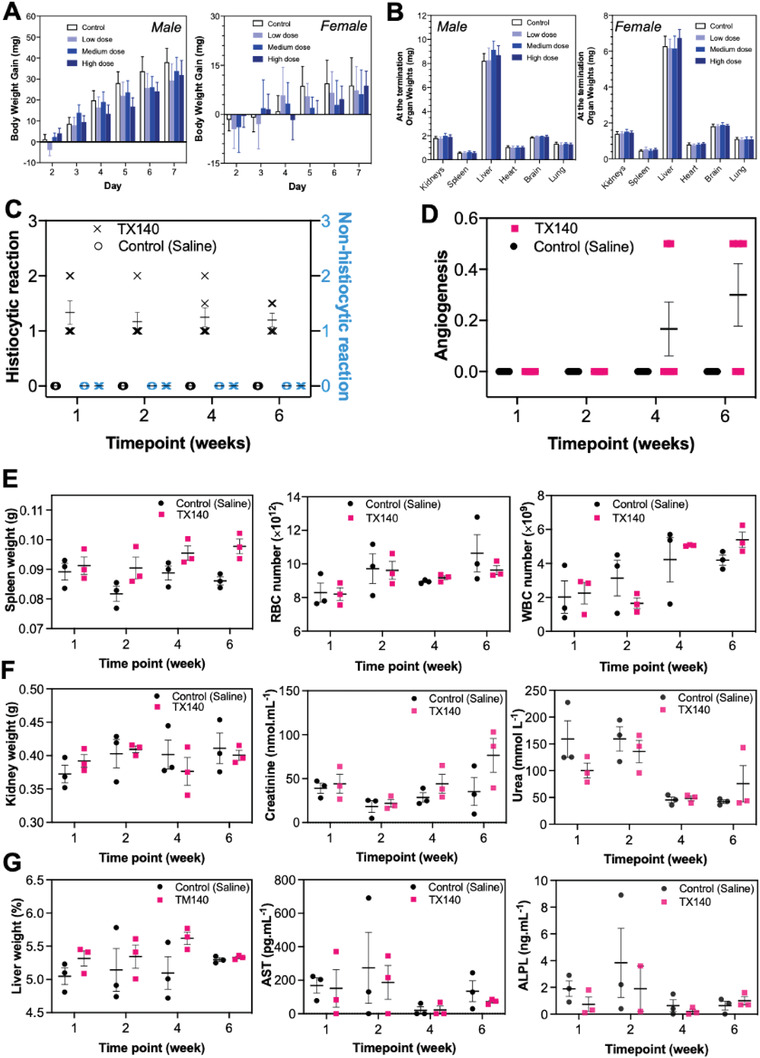
Acute toxicity, local, and systemic biocompatibility assessment of TX140. A) Body weight gain of female and male rats by polar and nonpolar extracts of TX140 during a week for acute toxicity assessment (n = 80, 40 female and 40 male animals). B) Organ weights of female and male rats by polar and nonpolar extracts of TX140 at the termination of the acute toxicity assessment (n = 80, 40 female and 40 male animals). C) inflammatory response (histiocytic and nonhistiocytic responses) to TX140 and normal saline at different time points. D) local angiogenesis at TX140 and normal saline injection site at different time points. E) spleen weight, red blood cell (RBC) and white blood cell (WBC) counts of TX140 and normal saline injected animals at different time points. F) kidney weight and kidney biochemistry markers (urea and creatinine) of TX140 and normal saline injected animals at different time points. G) liver weight and liver biochemistry markers of TX140 and normal saline injected animals at different time points (aspartate aminotransferase (AST) and alkaline phosphatase (ALP)). n = 24 in total, 6 at each time point for measurements reported in (C)–(G).
