Figure 5.
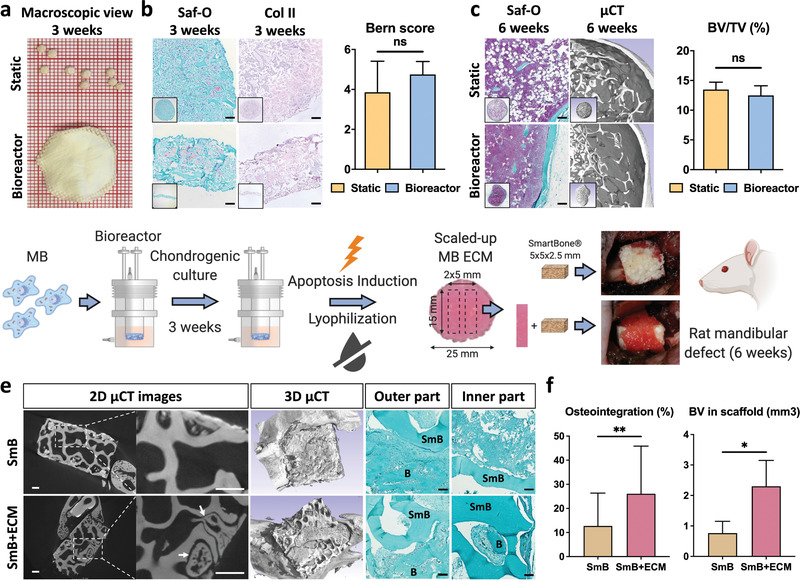
Scaled‐up, bioreactor‐manufactured human cartilage grafts enhance bone repair at an orthotopic site. a,b) Lyophilized tissues resulting from the static (top) or bioreactor culture (bottom) displayed a similar cartilage quality, as assessed histologically (Saf‐O and collagen II stainings) and by Bern scoring (n ≥ 4). c) The static and bioreactor‐generated tissues exhibited a similar osteoinductive performance in an ectopic subcutaneous model (histology analysis and µCT quantification of bone volume). To ensure a reliable bone‐to‐volume comparison, tissues of a similar dimension were implanted by punching out samples from the bioreactor‐derived cartilage discs. d) MSOD‐B (MB) cells are dynamically seeded, chondrogenically differentiated and subsequently induced to apoptosis within the bioractor device. Resulting tissues are lyophilized prior to their in vitro and in vivo evaluation. The bioreactor‐engineered cartilage repair capacity was evaluated in a nude rat mandibular defect model. The SmartBone (SmB) material was implanted alone or in combination with 5 × 15 mm bands cut out of the large 25 mm cartilage disc (SmB + ECM). e) After 6 weeks in vivo, extensive new bone formation (B) in both the inner and outer regions could be observed in the SmB + ECM implants. In constrast, SmB displayed a limited new bone formation, confined in the outer region. Scale bars = 500 µm for µCT and 100 µm for Saf‐O. f) Quantitative assessment of osteointegration and new bone volume formation confirmed the superior repair resulting from the SmB + ECM grafts (n = 3). The graphs represent mean + SD. *p ≤ 0.05, **p ≤ 0.01 determined by two‐tailed unpaired t‐tests.
