Figure 3.
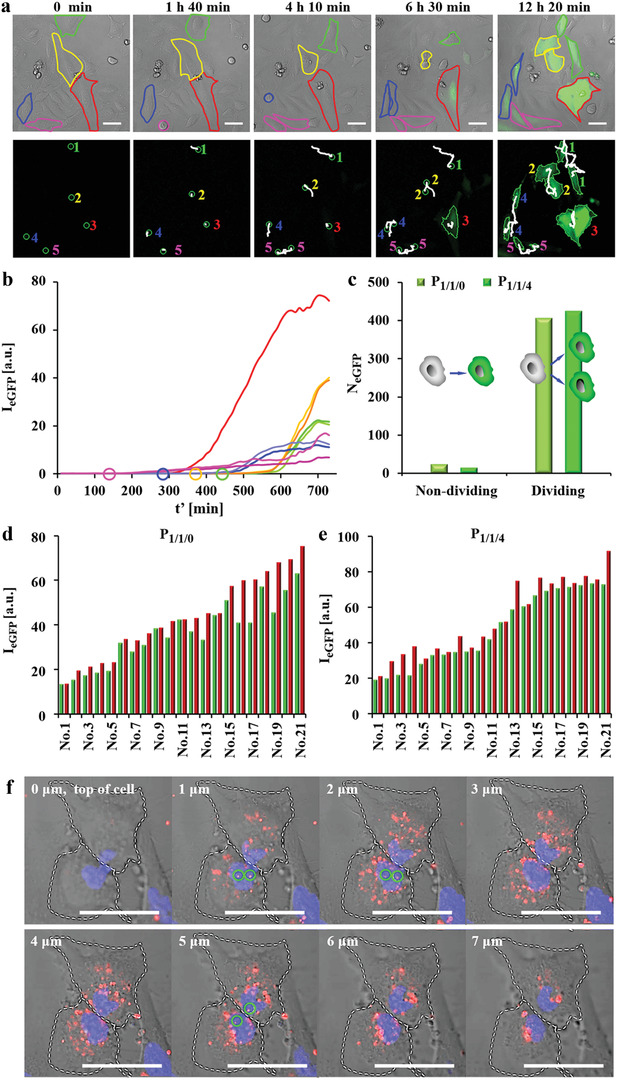
Time lapse images of eGFP expression in HeLa cells upon exposure to P 1/1/0 and P 1/1/4 polyplexes. Cells were incubated with polyplexes at 1.4 µg mL−1 peGFP in serum free medium for t = 3 h. Afterwards, cells were cultured in fresh 10% FBS supplemented cell culture medium and were imaged by CLSM every 10 min (the time point t′ = 0 refers to the start of imaging). a) Representative real‐time tracking of eGFP expression after P 1/1/0 transfection. The red outline indicates a non‐dividing cell. The green, yellow, blue, and pink outlines indicate cells that had divided during observation. The expressed eGFP signal is shown in the green fluorescence channel. The scale bars represent 50 µm. Cell tracking is shown in the lower panel. Here, the cell trajectories are shown in white. Daughter cells are indicated with the same number as their respective parent cells. b) Kinetics of eGFP expression (in terms of eGFP fluorescence intensity I eGFP) as calculated from the cells shown in (a). Circles indicate the time when cells started to divide. c) Statistics of non‐dividing and dividing cells expressing eGFP. N eGFP is the number of observed cells expressing eGFP, which had or had not divided during the observation period. d,e) eGFP expression of 21 pairs of daughter cells at the end of observation (t′ = 24 h) after transfection by d) P 1/1/0 and e) P 1/1/4 (for each pair of fluorescence the intensity of one daughter cell is shown in green, of the other daughter cell in red). f) Z‐stack images of P 1/1/0 polyplex distributions in the nuclei of two daughter cells just after mitosis. Cells were incubated with P 1/1/0 polyplexes prepared from DNACy5. Circles indicate DNA that was in the nucleus, as evidenced by orthogonal views (Figure S1.3.6.2, Supporting Information). The DNACy5 fluorescence is shown in red. The nuclei were stained with Hoechst 33342 and can be seen in the blue fluorescence channel. The scale bars represent 50 µm.
