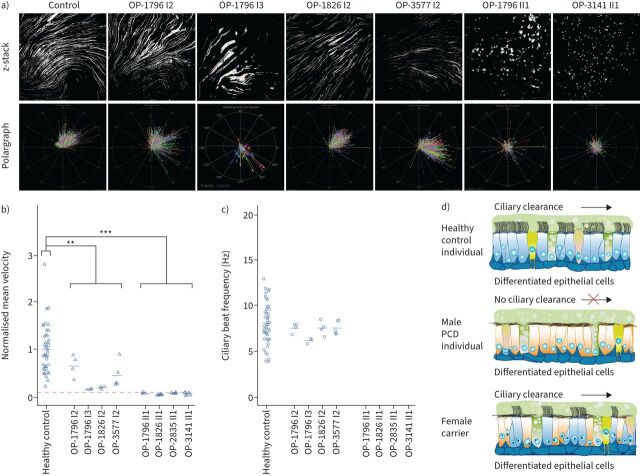
FIGURE 7.
Air–liquid interface cultures of respiratory epithelial cells from female carriers with heterozygous pathogenic DNAAF6 variants are able to generate a directed fluid flow while respiratory epithelial cells from male individuals with hemizygous pathogenic DNAAF6 variants are unable to produce a fluid flow. a) After complete cell differentiation and ciliation (30 days after airlift), fluorescent particles were added to the apical compartments of the respiratory epithelial cells to assess ciliary clearance capacity. Tracking videos are represented as z-stack projections, while the transport direction of each particle is summarised in polargraphs. b) Respiratory cells from a control group (n=19) and female carriers with heterozygous pathogenic DNAAF6 variants (OP-1796 I2, OP-1796 I3, OP-1826 I2 and OP-3577 I2) transported fluorescent particles in a linear direction along the cell layer. The velocity of this flow was significantly slower than that of the healthy controls (p=0.009, t-test). In contrast, the particle transport was non-oriented and significantly slower in male primary ciliary dyskinesia (PCD) individuals carrying hemizygous pathogenic DNAAF6 variants (OP-1796 II1, OP-1826 II1, OP-2835 II1 and OP-3141 II1; p=0.0003, t-test). Circles indicate values measured with 2 µm beads while triangles indicate values measured with 0.5 µm beads. Because two different bead sizes were used for the analyses, values were normalised against the mean value of the healthy control group. Exact values are shown in supplementary figure S15. The significantly reduced particle transport measured in the male PCD individuals (dashed red line) is a thermal-driven background flow (Brownian movement). c) Measurement of the ciliary beat frequency of healthy controls (8.5 Hz), female carriers with heterozygous variants in DNAAF6 (OP-1796 I2=7.6 Hz, OP-1796 I3=6.3 Hz, OP-1826 I2=7.7 Hz, OP-3577 I2=7.5 Hz) and male PCD individuals with hemizygous pathogenic DNAAF6 variants (0 Hz). There was a subtle reduction in the ciliary beat frequency in female carriers with heterozygous DNAAF6 variants compared to healthy controls (p=0.04, t-test). In total, 30 videos per individuals were analysed for statistical evaluation and 253 particles were tracked per video on average from three different cell culture inserts from each individual. d) Schematic summarising the particle tracking results. Ciliated cells from female control individuals (blue) were able to produce a directed flow. In contrast, in male PCD individuals with pathogenic DNAAF6 variants (orange), all cells display cilia immotility due to lack of functional outer and inner dynein arms and the absence of directed particle transport.